Summary:
Zebrafish are a commonly used model organism in the rare disease space, thanks to their significant advantages such as their affordability and ability to produce data quickly. Soon, zebrafish could become a fundamental resource in the drug discovery process more broadly, as regulatory bodies such as the FDA become more encouraging of alternative model use, and as larger pharmaceutical developers are wanting to benefit from these models too (Han, 2023). This article will discuss the suitability of zebrafish for efficacy testing, and how these fishes can help bridge the gap between in vitro models and the more traditional mammalian models in order to generate the data needed for the necessary steps of bringing a drug to market.
The zebrafish model has skyrocketed in use since it was first introduced as a research tool in 1972 by UO Professor George Streisinger – becoming a favorite model for rare disease researchers who are able to use it to investigate disorders which don’t have the resources of other, larger, pharmaceutical-funded studies (Varga, 2016). Zebrafish have also become popular for academic research labs worldwide, and are currently used in over 600 labs. This being said, the majority of larger pharmaceutical drug developers still use traditional mammalian models which have a few main drawbacks.
Drawbacks of mammalian models for drug development
- Longer timeline – long animal lifespan
- Cost – husbandry for mammals is extensive
- Paying for and negotiating licensing – for some disease indications the models will be licensed by an institution, and this can become costly and complicated
- Need to justify the 3Rs – especially as FDA is making a concerted effort to have research reduce the number of animals needed
Taken together, these drawbacks mean that pharmaceutical companies can not test more than a few compounds on their mammalian models, which, in turn, produces fewer drug leads. In part, to accelerate drug development, Congress passed the FDA Modernization Act which no longer makes animal testing necessary prior to clinical trials. This bill explicitly states that not only is the FDA now encouraging researchers to utilize non-mammalian animal models, but also to employ in cell cultures and computer modeling. While these models satisfy the 3Rs and are lower cost alternatives, as these studies look at a compounds’ effect in a very specific, isolated way – they then need to be followed up with animal models. This where a multimodal approach is needed, because, say you are using a computer model to simulate drug interactions and generate potential targets for new therapies – this model can run hundreds of drugs at a time, but, as previously mentioned, the study would have to quickly narrow, as it would only be feasible for the drug developer to test only two or three of these compounds in a mammalian mice or rat model. Thus, zebrafish have the potential to become a fundamental resource in the overall process of discovering new drugs, as they can act as a midway model, allowing for screening a larger pool of potential targets prior to conducting costly experiments on other models.
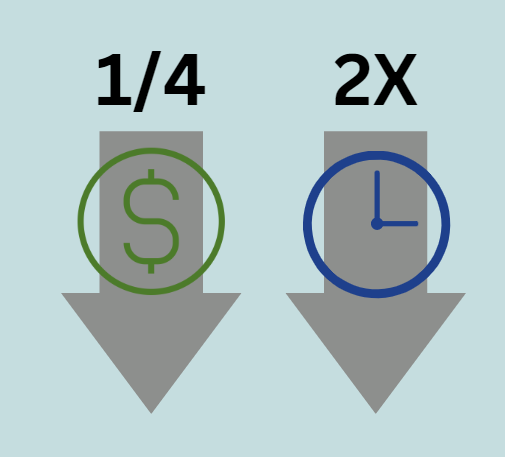
Figure 1. Drug candidate screenings using zebrafish are 2x fast and ¼ cost compared to mammalian mice models.
How the Tide is Turning to Zebrafish
Beyond governmental efforts to promote the increased usage of alternative models such as zebrafish in drug development, non-profit institutions themselves are working to make non mammalian models the standard for the early decision making stage of drug discovery. For instance, Mount Desert Island Biological Laboratory launched an initiative last year that seeks to smooth the regulatory process for using zebrafish and C.elegans in early drug discovery stages (Mesa, 2022) The Director of MDI Bioscience, Jim Strickland explained that they’re, “building this organization from the ground up with an eye toward the future (…) with the goal that eventually, as these models become regulatory models” with easily to follow specific guidelines, which is the current status for mice, rats, and dogs as defined by the ICH [International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use].
Non-Mammalian models (..) can help pharmaceutical companies better characterize early-stage molecules, and be able to quickly select those molecules with the greatest promise before more significant investment is made in mammal studies
Jim Strickland, Director of MDI Bioscience
The lack of streamlined protocols for non mammalian models, such as zebrafish, is one of the most commonly touted reasons for pharmaceutical companies’ hesitancy to adopt the model, however, researchers such as Dr Esguerra thinks that this will soon change as more literature is being published utilizing zebrafish. Moreover, Dr Esguaerra, the Zebrafish Core Faculty Leader at the University of Oslo, believes that some of the zebrafish’s value is in its ability to act differently than a mammalian model would (Repasky, G. (2022). Dr Esguarra brings a unique outlook on drug discovery, as has moved between academia and industry (biotechnology); she explained that “to truly add value [by using zebrafish. They needed to] not just copy what was being done in the mouse.” For instance, modeling the mechanisms of neurodevelopmental disorders during early brain development is notoriously difficult in mice models, however, it is possible in fish thanks to zebrafish’s ex utero development and transparent embryos.
Nearly everyone that I collaborate with now, has been skeptical initially about the zebrafish model. It's not a warm blooded, furry animal. But once they get to know the model and saw the power and versatility of what it can achieve, they are converts. They're hooked. I have had collaborators now for over a decade.
Dr Camila Esguerra, Zebrafish Core Faculty Leader, University of Oslo
Conclusion:
In summary, the increasing use of zebrafish and other non-mammalian models in drug development, coupled with ongoing efforts to streamline protocols and establish regulatory guidelines, indicates a turning tide. These models offer pharmaceutical companies the opportunity to better characterize early-stage molecules and select promising candidates before investing in more extensive mammalian studies. Zebrafish, with its unique attributes and ability to bridge the gap between high-throughput screening and costly mammalian experiments, has the potential to become an indispensable resource in the quest for discovering new drugs.
Contact our experts to learn about how we can provide you with the efficiency data that you need.
References:
Han J. J. (2023). FDA Modernization Act 2.0 allows for alternatives to animal testing. Artificial organs, 47(3), 449–450. https://doi.org/10.1111/aor.14503
Varga, M (2016), The Doctor of Delayed Publications – the remarkable life of George Streisinger, https://thenode.biologists.com/doctor-delayed-publications-remarkable-life-george-streisinger/careers/
Wadman, M (2023). FDA no longer needs to require animal tests before human drug trials, Science, Vol 379, 6628, https://www.science.org/content/article/fda-no-longer-needs-require-animal-tests-human-drug-trials#.Y76rZYPSO3g.twitter
Paul, S. (2023). Dr. Paul’s Bipartisan FDA Modernization Act 2.0 to End Animal Testing Mandates Included in 2022 Year-end Legislation, Dr Rand Paul, US Senator, Kentucky. https://www.paul.senate.gov/news/dr-pauls-bipartisan-fda-modernization-act-20-end-animal-testing-mandates-included-2022-year
Mesa, N (2022). Oust the Mouse: A Plan to Reduce Mammal Use in Drug Development, The Scientist, https://www.the-scientist.com/news-opinion/oust-the-mouse-a-plan-to-reduce-mammal-use-in-drug-development-69805
Repasky, G (2022). Meet Camila Esguerra: Converting skeptics to believers on the versatility of zebrafish models, The Centre for Molecular Medicine Norway



