Cutting-Edge Zebrafish CRISPR Knock-In Services
InViVo offers Zebrafish CRISPR Knock-In service that utilizes the CRISPR/Cas9 gene editing technology to introduce specific genetic modifications into zebrafish embryos. This service allows researchers to precisely insert desired genetic elements, such as point mutations or specific gene sequences, into the zebrafish genome. Our scientists can help you design your next disease model to your precise specifications using cutting-edge CRISPR techniques.
More Zebrafish CRO Services
Why Choose InVivo's Zebrafish Crispr Knock-in Service?
- Expertise: Our experienced scientists specialize in zebrafish CRISPR technology and knock-in techniques, ensuring precise and reliable genetic modifications.
- Customized Approach: We offer personalized strategies tailored to your specific research goals and requirements.
- High Success Rate: Our optimized protocols and advanced methodologies result in a high success rate for generating zebrafish lines with desired genetic modifications.
- Quality Control: We maintain strict quality control measures to ensure accurate targeting and efficient incorporation of genetic modifications.
- Timely Delivery: Our efficient workflow enables us to deliver your custom zebrafish models within agreed-upon timelines.
- Comprehensive Support: We provide ongoing technical assistance and guidance to help you achieve your scientific objectives.
Zebrafish Crispr Knock-in Techniques
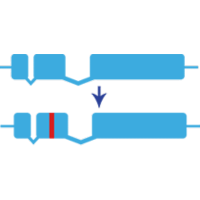
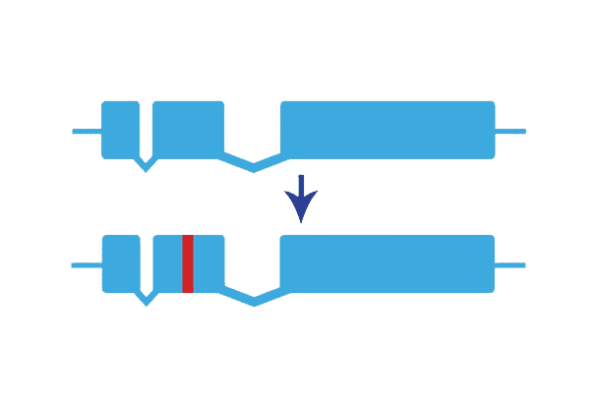
Gene Knock-in Technology is a powerful tool to test the function of specific domains in a protein or to model known human mutations in research animals. These small precise changes in specific amino acid sequence can be used to better understand how gene mutations are associated with human health and disease.
We can successfully repair a point mutation by knocking in a wild-type repair template to restore gene function. Below is a visual readout of knock-in repair in F0 (injected, generation zero) zebrafish larvae carrying a point mutation in a gene essential for pigmentation. To determine if this repair has been transmitted through the germline, we would subsequently raise these F0 animals to adulthood and screen their progeny for wild-type development of pigment cells. Images were taken at 48 hours post-fertilization (hpf).
Knock-in techniques in zebrafish utilize CRISPR/Cas9 to insert small sequence changes at a specific site in the zebrafish genome. sgRNA site(s) are selected to open the genome adjacent to the desired edit, and a repair template is used to introduce precise changes in the genome. Animals are then raised to adulthood and screened for transmission of the desired edit.
- Knocking in point mutations. Specific coding sequences can be changed to precisely match genetic sequence variants seen in human disease.
- Knocking in a fluorescent tag at the native locus. Observe when and where your gene of interest is expressed using fluorescence. A genetically encoded fluorescent marker (GFP, mCherry, etc.) can be inserted in-frame for tagging of either the N- or C-terminus of your protein.
- Knocking in LoxP sites. Insertion of two LoxP sites flanking your gene of interest creates the ability to eliminate the gene of interest with expression of the Cre recombinase. Cre recombinase can be expressed using tissue or stage-specific promoters for precise control of gene function. Learn more>>.
- Knocking in a protein tag. Genetically encoded tags (FLAG tag, HA-tag, poly(His) tag, etc.) can be inserted in-frame (up to 150bp) for tagging of either the N- or C-terminus of your protein. The protein can then be observed by Western blots, immunofluorescence, and immunoprecipitation using commercially available common antibodies.
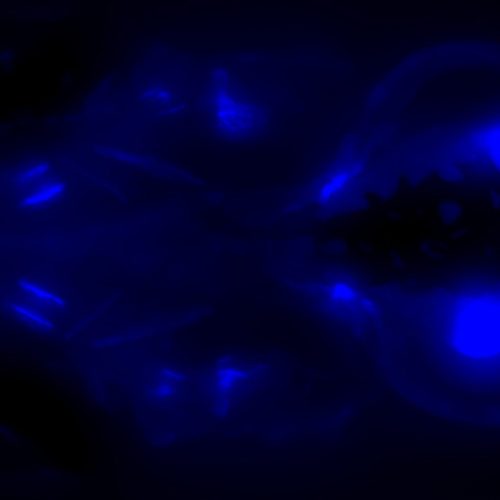

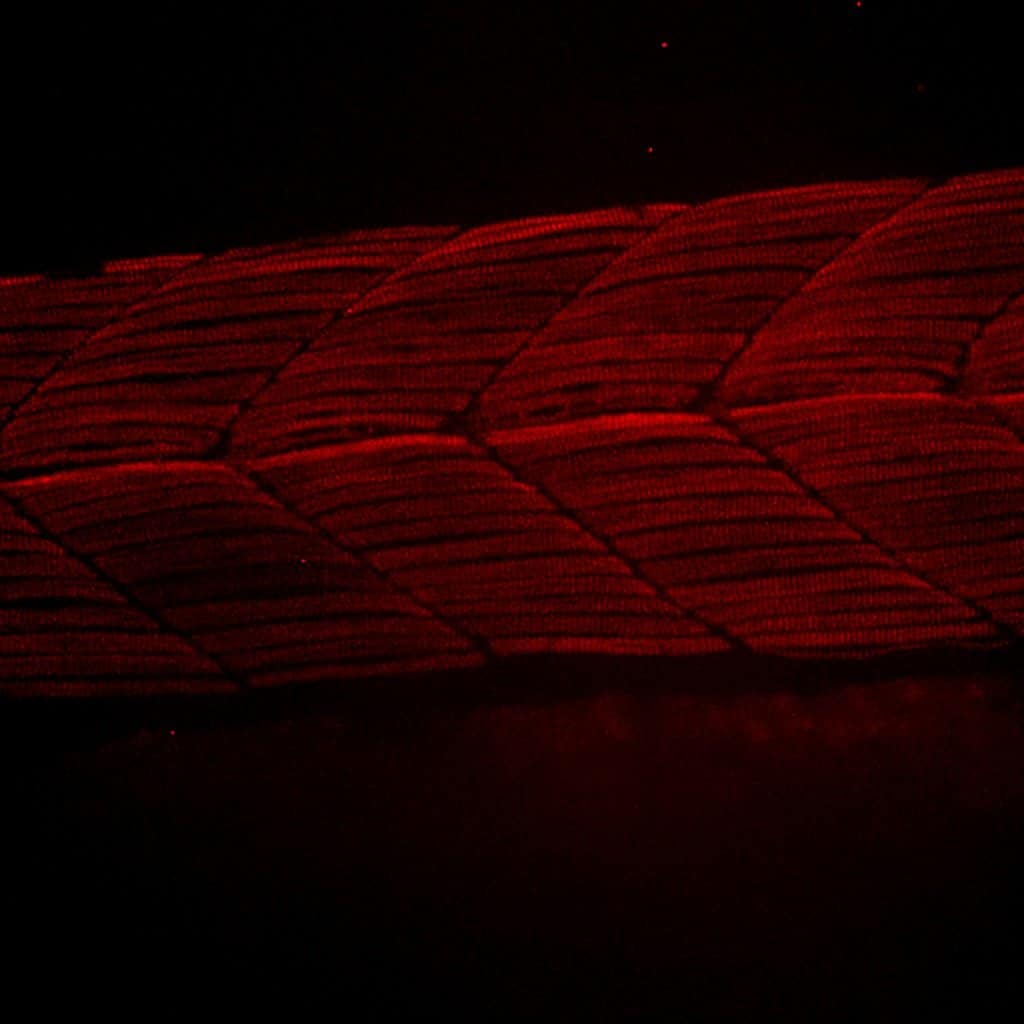
(Left): SelN-BFPSTOP with BFP followed by a STOP codon inserted into the N-terminus of Selenon (SelN). (Center and Right): Ryr1a-mCherry line where the endogenous Ryr1a was tagged with mCherry at the N-terminus. Image courtesy of Melissa A. Wright, MD/PhD, Assistant Professor of Pediatric Neurology at University of Colorado. Read the customer story.
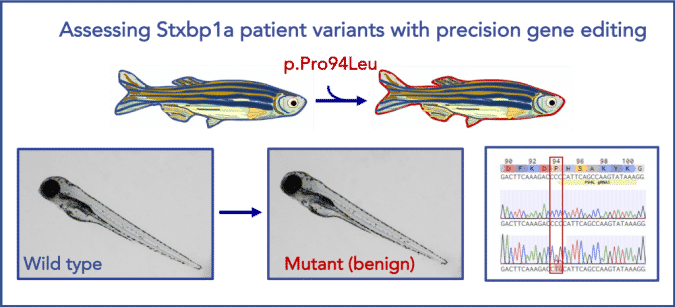
Modeling STXBP1 Patient Variants in Zebrafish
Illustration of a successful precise point mutation of stxbp 1a in zebrafish. stxbp1a is a highly conserved zebrafish ortholog of human STXBP1 (87% identity). Using CRISPR/Cas9 technology, we were able to precisely generate a benign patient mutation at the conserved amino acid residue (CCC>CTG, p.P94L).
Keys to a Successful Knock-in in Zebrafish
Know your gene
Good knowledge of your gene, alternate isoforms expressed and related orthologs is required for designing an efficient experimental strategy.
Choose good sgRNAs
To ensure you use an sgRNA that guides efficient cutting, 2-3 sgRNAs with different recognition and PAM sites should be designed for each knock-in experiment
Validate sgRNAs
All sgRNAs should be validated in vivo to ensure that they are not toxic and that they efficiently guide Cas9 cutting of the DNA. Read our article on why validation is important.
Create the right donor DNA template
The size of your knock-in insert determines the type of template (double-stranded vs. single-stranded) and the size of the homology arms used.
Design and test primers
Developing a robust assay to detect your edit is critical when you begin screening the embryos produced by the F0 founders.
Screen, Screen, Screen
Knock-ins are notoriously low efficiency, and on top of that, the F0 germline is mosaic and can transmit multiple edits. The rate of transmission to your F1 generation will vary depending on when the F0 germline edit was made in development. Ranges from 3% to 50% have been observed. Be sure to grow enough F1 animals that you will be able to find your F1 heterozygotes.
CRISPR Knock-in Service Options
Standard Injection Mix
Validated sgRNA Injection Mix
Fully Validated Injection Mix
Mosaic Clutch* (F0 injected embryos)
Full Build** (Sequence verified hetero zygous line)
KI Design
Injection Mix Assembly
Locus Evaluation & Sequencing Primers
in vivo sgRNA Testing
in vivo Editing Assessment & Screening Reagents
Expertly Injected Embryos
Germline Transmission Screening & Line Propagation
* This service is only available to clients in the United States.
** Full Build includes screening n=100 adults; standard husbandry charge applies.
Injection Mix And SgRNA Validation Details
Standard Injection Mix
Choose this option if you want us to pick your sgRNA site, have already tested your sgRNA in vivo, or want to make a CRISPR knockout. We send a custom designed injection mix and you develop your own preferred detection/screening protocol.
What’s Included
- Consultation with a genetic engineer to develop the genome editing strategy
- In silico design of all reagents including the sgRNA site and donor homology template
- Sequence files for the donor homology construct and edited locus
- 4 vials of 10uL lyophilized sgRNAs duplexed with the Cas9 protein
- Injection dyes for early visualization of injection success
- Detailed Instructions on mix reconstitution and injection protocols.
Validated sgRNA Injection Mix
Choose this option if you want us to guarantee your injection mix is designed around a high efficiency sgRNA site using our rigorous in-house testing process.
What’s Included?
- OUR COMPLETE VALIDATED INJECTION MIX PLUS:
- Expertly injected embryos for direct submission to you nursery
- Fully validated PCR primers and protocols for edit detection assay in your own lab.
Standard Injection Mix + Screening Primers
Choose this option if you want to inject and screen for your edit by PCR without having to worry about designing your own screening primers. This option includes our standard mix plus ready-to-go screening primers.
What’s Included?
- OUR STANDARD INJECTION MIX PLUS:
- Prescreened Allele Specific PCR primers to detect your edit in zebrafish tissue.
Complete Validated Injection Mix
Choose this option if you want a CRISPR knockin with completely validated reagents to use in your lab and to find your gene edited line. This is the best Custom Injection Mix option for the novice zebrafish line builder.
What’s Included?
- OUR VALIDATED sgRNA INJECTION MIX PLUS:
- Full in vivo evaluation of somatic editing efficiency.
- Confirmation of integration capacity of your edit into the zebrafish genome
Mosaic Clutch (F0 injected embryos)
Choose this option if you want a CRISPR knock-in with completely validated reagents to use in your lab and to find your gene edited line. This is a good option for the researcher who wants to outsource part of the workload in the development of a new line.
What’s Included?
- OUR VALIDATED sgRNA INJECTION MIX PLUS:
- Full in vivo evaluation of somatic editing efficiency.
- Confirmation of integration capacity of your edit into the zebrafish genome
Full Build (Sequence verified heterozygous line)
Choose this option if you want us to deliver a stable sequence validated CRISPR knock-in line.
What’s Included?
- VERIED CLUTCH (F0 INJECTED EMBRYOS) PLUS:
- Growth of F0 injected embryos in our nursery
- Rigous screening of F0 injected adults for germline transmission of your edit
- Sequence conformation and propagation of F1 heterozygous animals
Component Descriptions for our Injection Mix & sgRNA Validation Service
Injection Mix
We create a mix using quality custom reagents from our validated suppliers. The sgRNAs are duplexed with the Cas9 protein.
The mix is created using concentrations of Cas9, sgRNA and donor homology that work well in our genome editing pipeline. The mix is provided as a stable dehydrated reagent.
Four injection mixes are provided that reconstitute to 5ul of mix each.
- Custom reagents from validated suppliers
- Four 10uL lyophilized injection mixes with instructions on reconstitution
- Rhodamine dye for early visualization of injection success
Screening Reagents
You can miss your edit if you don’t have a good assay to detect it.
We will design and test a robust assay to detect your edit, which is critical when you begin germline screening.
We will also provide PCR primers and protocols for an edit detection assay.
- PCR primers designed and tested to identify the target edit
- PCR protocol to identify animals that contain your edit
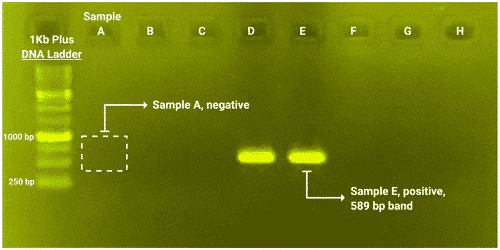
sgRNA Testing
Ensure that you have the quality reagents you need for a successful genome edit. The efficiency of the sgRNA guided cutting can vary widely and is directly tied to the achievement of a successful genome edit.
Algorithms and experience help us choose the best candidate sgRNAs for your project, but the guided cutting efficiency of an sgRNA can be impacted by genome organization, for example, chromatin accessibility. The best way to determine the effectiveness of an sgRNA in a Zebrafish genome is to test it in vivo in the injected embryos.
We test your sgRNAs for their ability to guide Cas9 cutting. Our process also identifies SNPs and polymorphoisms in your locus that may impact homology directed repair. Embryos are injected with two different sgRNA/Cas9 duplexes and we determine the cutting efficiency for each sgRNA. If both sgRNAs fail to guide successful cutting, a third sgRNA is injected and tested.
Additional sgRNA testing can also be added with consultation.
- Up to 3 sgRNAs tested for CRISPR cutting efficiency in zebrafish embryos
- Selection against sgRNAs that have toxic effects
- Identification of an sgRNA that is successful for guiding CRISPR cutting
In Vivo Editing Efficiency Screening
Have total peace of mind by having us test your injection mix in our facility.
Our skilled injectionists will inject embryos with your mix and determine the somatic integration efficiency by PCR.
We will confirm the precise edit by sequencing to ensure that your edit is occurring correctly with no erroneous insertions.
- Test injection of mix into embryos
- Integration efficiency in injected embryos
- Sequencing to confirm precise edit
Expertly Injected Embryos
Our team precisely injects the sg(s) targeting the locus or region of interest, Cas9 protein and the donor homology template to generate F0 embryos.Germline Transmission Screening & Line Propagation
F0 embryos are reared to adulthood and then screened for germline transmission using a combination of screening techniques to identify germline founders. Identified adults are propagated to ensure a sufficient number of animals to ship to your facility.More Zebrafish Gene Editing Services
Key References
A thorough review of current methods around point mutation knock-ins in zebrafish using CRISPR/Cas9, including the detailed description of the screening workflow for identifying the rare precise editing events generated with current knock-in approaches.
An excellent overview of knock-ins in zebrafish. The article provides one of the most comprehensive side-by-side comparisons of donor template designs and knock-in strategies available for zebrafish at the time. A detailed discussion of the different repair pathways, template designs, and different reagent choices, as well as their limitations and paths forward for improving knock-in editing efficiencies in the future is very informative
Boel et al. delve into the dizzying realm of ssODN-mediated knock-in repair with a comprehensive assessment of the impacts of different repair templates and design strategies on editing outcomes. Potential mechanisms of repair that lead to complex mutation patterns obtained with ssODN templates, as well as potential avenues for improvement on these methods are also discussed.
Ready to get started?
Ready to connect with us to learn more about working with our company or our technology?
Submit your inquiry below & we will get back to you soon.