We recently were asked to procure a special zebrafish diet, which got us thinking about zebrafish diets overall. Once we started researching this custom diet, we realized that the zebrafish diet is more complicated than one might think. There are many considerations that go into a zebrafish diet, as it is not yet standardized. Currently, rodent feed is standardized (AIN-93) and there are strides being made to standardize the diets of zebrafish (Nielson 2018).
Benefits of Standardizing Diets
Rodent diets have been standardized since 1993 when the AIN-93 diet was first established, creating uniformity throughout labs (Nielson 2018). There are a variety of benefits including the benefit of rodent diets, which can be reproduced easily. This reproducibility allows researchers to manipulate components of the diets and in turn isolate the effects of these changes resulting in more accurate studies in the realm of metabolic diseases. Additionally, the AIN-93 diet has reduced the variability among rodent diets around the world, resulting in better comparison of experimental outcomes and yielding greater confidence in the results produced (Watts & D’Abramo 2021). Finally, standardized diets are very cost effective, saving researchers money.
While rodent diets have been standardized for many years, zebrafish diets have yet to be standardized. One of the main reasons for this is due to the variability in feeding patterns of zebrafish during the different life stages; as well as, the number of closed formula diets utilized in lab settings.
Lack of standardization in diets can result in weight differences, inconsistent embryo production, and even delays or early onset sexual maturity, thus having a bigger impact on experimental results then one might expect. Standardization of the zebrafish diet, similar to the AIN-93 in mice, could also open up research on the influence of nutrients and how diet affects the expression of genes and metabolic diseases.
General Overview of Diet Standards
So, what makes a diet standardized? There are a few main components that go into a standardized diet. Primarily, it must contain all necessary nutrients for zebrafish growth including protein, lipids, carbohydrates, vitamins and minerals. While each one of these components is crucial, we will be focusing on macronutrients, and will be comparing two common laboratory zebrafish diets to this recommended diet.
Currently, there are two well-known zebrafish foods that we will be referencing in this piece – Gemma Micro and Zebrafeed. In addition, many labs choose to make their own zebrafish food, which allows for food tailored to their specific research needs, but increases the chance of variability across labs.
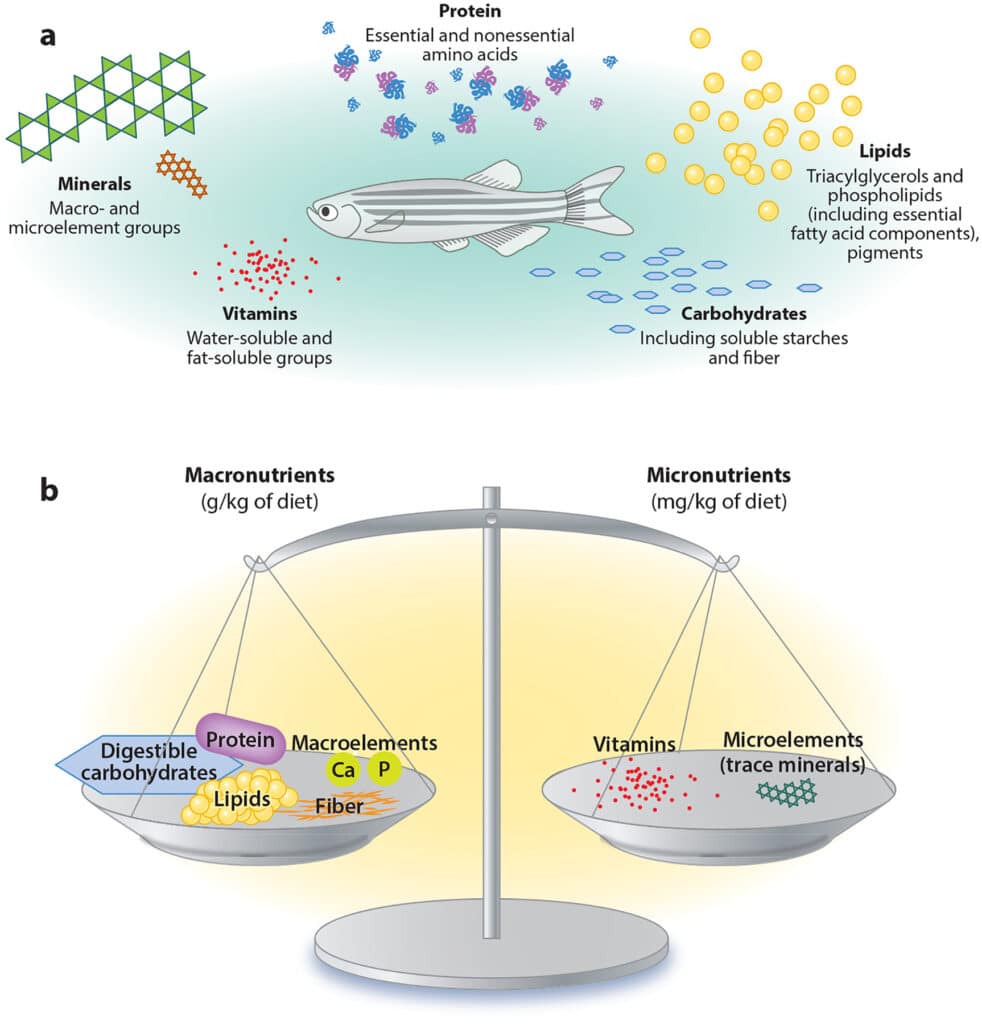
Figure 1. (a) Sources and (b) relative inclusion levels of dietary nutrients in a standard reference diet for zebrafish. Despite the large compositional volume (mass) of macronutrients in a reference diet, micronutrients must be balanced to ensure proper metabolic function and efficiency. Abbreviations: Ca, calcium; P, phosphorus (Watts & D’Abramo, 2021).


Image 1, 2. Examples of zebrafish feed (Sparos, n.d.; zebcare, n.d.).
Components of a Standardized Diet
First up is protein. A recent paper, which used fishmeal as the protein source, stated that the ideal amount of protein in a juvenile zebrafish diet is 37.6% for maximum weight gain and 44.8% for maximum protein retention (Fernandes et al. 2016). Both Zebrafeed (59%) and Gemma (60%) are above these recommended protein levels. When it comes to protein, it has also been suggested that no more than two purified protein sources should be used in a standardized diet (Watts & D’Abramo 2021).
Lipids are another macronutrient component of a zebrafish diet that are included to provide metabolic energy and essential fatty acids (Watts & D’Abramo 2021). While research on the specific requirements of lipids in a zebrafish diet is limited, other species with similar diets require between 10% to 18% of their total diet to be lipids (NRC 2011). It is also recommended that multiple purified sources of lipids, versus single sources, from plants or animals be used to reach essential fatty acid requirements. Both Zebrafeed (12%) and Gemma (14%) are within these recommended lipid levels.
Carbohydrates are the third macronutrient, and one that needs additional research in zebrafish. There is little info available about the ideal carbohydrate composition of dry feed, and surprisingly the percentage of carbohydrates in Zebrafeed and Gemma is not provided (except for starch making up 1.9% of Gemma feed), but there are several ingredients in the list which include carbohydrates such as wheat gluten and maize starch. Robison et al., tested 0%, 15%, 25%, and 35% carbohydrate diets, and found that a 0% carbohydrate diet resulted in significantly lower growth than the higher carbohydrate diets, suggesting that carbohydrate content can have a big impact on general health.
Both Gemma Micro and Zebrafeed have similar nutritional analysis compared to the recommended diet structure for zebrafish. As research occurs and new information becomes available, it would be great to see updates to both Gemma Micro and Zebrafeed formulations based on empirical findings.
Conclusion:
While rodent diets have been standardized for nearly 30 years, zebrafish diets still have a long way to go before a standardized diet can be published. This article highlights the need for more research around zebrafish feed requirements in order to create a standardized diet. Once a standardized diet is established, this will allow research to be more reproducible, decrease variability between facilities or labs, and even save zebrafish labs money.
References
- Fernandes, H., Peres, H., & Carvalho, A. P. (2016). Dietary Protein Requirement During Juvenile Growth of Zebrafish (Danio rerio). Zebrafish, 13(6), 548-555. https://doi.org/10.1089/zeb.2016.1303
- Forrest H Nielsen, 90th Anniversary Commentary: The AIN-93 Purified Diets for Laboratory Rodents-The Development of a Landmark Article in The Journal of Nutrition and Its Impact on Health and Disease Research Using Rodent Models, The Journal of Nutrition, Volume 148, Issue 10, October 2018, Pages 1667-1670, https://doi.org/10.1093/jn/nxy121
- NRC (Natl. Res. Counc. Natl. Acad.). 2011. Nutrient Requirements of Fish and Shrimp
- Washington, DC: Natl. Acad. Press
- Robison, B. D., Drew, R. E., Murdoch, G. K., Powell, M., Rodnick, K. J., Settles, M., Stone, D., Churchill, E., Hill, R. A., Papasani, M. R., Lewis, S. S., & Hardy, R. W. (2008). Sexual dimorphism in hepatic gene expression and the response to dietary carbohydrate manipulation in the zebrafish (Danio rerio). Comparative biochemistry and physiology. Part D, Genomics & proteomics, 3(2), 141-154. https://doi.org/10.1016/j.cbd.2008.01.001
- Watts, S. A., & D’Abramo, L. R. (2021). Standardized Reference Diets for Zebrafish: Addressing Nutritional Control in Experimental Methodology. Annual review of nutrition, 41, 511-527. https://doi.org/10.1146/annurev-nutr-120420-034809
- Sparos (n.d.). Nutrition in Aquaculture, Sparos website, https://www.sparos.pt/
- zebcare (n.d.). GEMMA MICRO ZEBRAFISH DIET, zebcare website, https://www.zebcare.nl/systemen/gemma-micro-zebrafish-diet/





