Thigmotaxis Overview
Thigmotaxis is a “wall hugging” behavior exhibited in both zebrafish and mice. Thigmotaxis has been used to model anxiety and stress response.
Zebrafish have been established as a good model of anxiety in humans.
Zebrafish offer the advantage of a high throughput approach to anxiety modeling.
- Relevant disorders for anxiety modeling: Social Anxiety, Panic Attacks, Phobias, Substance-induced Anxiety, Post Traumatic Stress Disorders.
Introduction
Ellen is enjoying that perfect moment, nestled with a book in the corner booth of her favorite cafe. Fragrant coffee fills her with warmth as the quiet chaos of the city envelops her. As she glances up for a from her book, the once-friendly space now closes in, and the air thickens with an foreboding weight. Sweat beads form on her forehead, and her hands tremble involuntarily. A tightening sensation in her chest intensifies as her pulse races, and Ellen is frozen by an inexplicable conviction that these quicking breaths might be her last.
Ellen is in the grip of an anxiety attack, a visceral experience shared by millions challenged with anxiety disorders. These enigmatic disorders, which include panic disorders, phobias, social anxiety, and others, stem from deeply encoded animal “fight or flight” mechanisms. Finding effective treatments requires an understanding of the intricate connections between genetics, neurobiology, physiology, and life experiences.
Traditional rodent models have played a pioneering role in advancing our knowledge, offering an extensive library of behavioral assays. However, their limitations in terms of being low-throughput, costly, and time-consuming have prompted a shift toward exploring alternative animal models. Zebrafish are a relative newcomer to the field and introduce the twist of the aquatic environment. Nonetheless, studies over the last few decades have defined conserved neurological processes between fish and mammals and solidified the validity of the zebrafish as a model for human behavioral disorders. The ability to perform many traditionally rodent assays in a small animal model quickly, economically, and ethically, offers new opportunities for accelerating understanding and therapeutic discovery.
Translating behavioral assays from a terrestrial to an aquatic environment is not an easy task and this series aims to bring awareness to alternative assays and guide researchers in selecting applicable zebrafish assays. In an earlier post HERE, we provided an overview of behavioral assays that were the most directly translatable between rodent and fish. In this blog post, we take a closer look at Thigmotaxis, one of the most salient conserved behaviors between mammals and fish, as it relates to anxiety disorders.
What is Thigmotaxis?
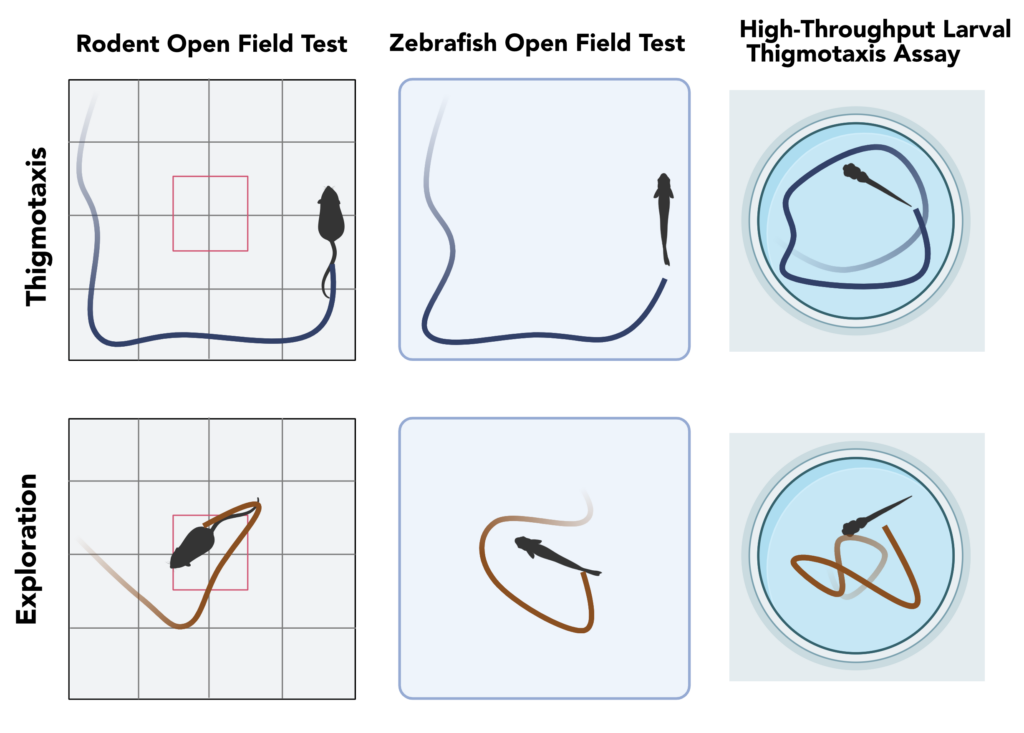
Thigmotaxis is sometimes referred to as “wall hugging” and is a well-validated indicator of stress and anxiety conserved across animal species. Many animals, including humans, exhibit decreased exploratory behavior and avoidance of open spaces when anxious or in an unfamiliar environment. In rodents, Thigmotaxis is assessed through the classic “Open Field Test” in which an animal’s movement is recorded as it explores an enclosure. In a new enclosure, animals will defensively linger closer to boundaries and objects until they become more familiar and begin exploring the open area. Applying a stressor before the test will increase the amount of time spent near the boundaries. The latency to resume exploration is a readout of the intensity of the stressor or the effectiveness of a potential treatment.
Zebrafish as an Anxiety and Stress Model
Thigmotaxis is one of the behaviors most directly translatable from mammals, as zebrafish will likewise cling to the walls of their enclosure, which could be a tank or well in a plate. In a behavior derived from thigmotaxis, zebrafish also dive to the bottom of the enclosure and remain there for a time proportional to their anxiety. This third dimension is assayed using a distinctive “Novel Tank Diving” assay, but here we discuss the horizontal thigmotaxis approach comparable to the rodent assays.
The validity of adult zebrafish thigmotaxis assays has been confirmed through studies replicating responsiveness to drugs that mediate anxiety as well as direct physiological correlation of cortisol levels with thigmotaxis behavior. With their short generation time and large brood size, Zebrafish solve one of the key dilemmas with rodent experimentation. Within an experiment, rodents and other animals become habituated to the environment, affecting their responsiveness in any subsequent behavioral assays. For mammals, the researcher faces the dilemma of either reusing experienced (i.e. compromised) animals or increasing animal turnover, massively expanding the research expenditure and time. In steep contrast, a large cohort of adult zebrafish can be produced in 2-3 months.
An even greater speed advantage is availed by the ability to assay thigmotaxis in larval zebrafish. Larvae can be generated on demand by the thousands, and by six days post-fertilization, these tiny fish exhibit characteristic thigmotaxis behavior, avoiding the center region of their vessel. Importantly, larval zebrafish mirror the activity of anxiety-modulating drugs observed in both adult zebrafish and rodents. The small size of larval zebrafish makes them compatible with multiwell plate formats, opening up a range of high-throughput screening possibilities. The wide availability of robust movement tracking software makes thigmotaxis in larval zebrafish a quantitative and scalable solution for therapeutic screening.
Conclusion
The versatility of zebrafish as an alternative model for studying anxiety-related behaviors is underscored by their ability to swiftly and economically replicate traditional rodent assays. The Thigmotaxis assays, both in adult and larval zebrafish, offer researchers powerful tools to explore anxiety mechanisms and drug responses with efficiency and ethical considerations in mind. As we dive deeper into the aquatic world of zebrafish research, exciting opportunities for advancing our understanding of anxiety disorders and accelerating therapeutic discoveries unfold. Stay tuned for more insights in our ongoing series on zebrafish as a valuable model in behavioral research.
References
Ahmad, F., & Richardson, M. K. (2013). Exploratory behaviour in the open field test adapted for larval zebrafish: Impact of environmental complexity. Behavioural Processes, 92, 88–98. https://doi.org/10.1016/j.beproc.2012.10.014
Collier, A. D., Kalueff, A. V., & Echevarria, D. J. (2017). Zebrafish Models of Anxiety-Like Behaviors (A. V. Kalueff, Ed.; pp. 45–72). Springer International Publishing. https://doi.org/10.1007/978-3-319-33774-6_3
Levin, E. D., & Cerutti, D. T. (2009). Behavioral Neuroscience of Zebrafish. In J. J. Buccafusco (Ed.), Methods of Behavior Analysis in Neuroscience (2nd ed.). CRC Press/Taylor & Francis. http://www.ncbi.nlm.nih.gov/books/NBK5216/
Noldus, L. P. J. J., Spink, A. J., & Tegelenbosch, R. A. J. (2001). EthoVision: A versatile video tracking system for automation of behavioral experiments. Behavior Research Methods, Instruments, & Computers, 33(3), 398–414. https://doi.org/10.3758/BF03195394
Schnörr, S. J., Steenbergen, P. J., Richardson, M. K., & Champagne, D. L. (2012). Measuring thigmotaxis in larval zebrafish. Behavioural Brain Research, 228(2), 367–374. https://doi.org/10.1016/j.bbr.2011.12.016
Stewart, A., Gaikwad, S., Kyzar, E., Green, J., Roth, A., & Kalueff, A. V. (2012). Modeling anxiety using adult zebrafish: A conceptual review. Neuropharmacology, 62(1), 135–143. https://doi.org/10.1016/j.neuropharm.2011.07.037
Vaz, R., Hofmeister, W., & Lindstrand, A. (2019). Zebrafish Models of Neurodevelopmental Disorders: Limitations and Benefits of Current Tools and Techniques. International Journal of Molecular Sciences, 20(6), Article 6. https://doi.org/10.3390/ijms20061296