Niemann-Pick Type C (NPC) is a rare developmental disorder affecting 1 in 150,000 children. Symptoms develop early in childhood due to a lysosomal storage disorder, worsen over time and, ultimately, lead to premature death.
Because it is an orphan disease, NPC requires a low-cost approach for drug development. There is currently no cure for NPC.
We recently began a research collaboration with Perlara, a scientific discovery company with a focus on finding treatments for rare diseases.
This collaboration is fascinating to us for two reasons: this is the first developmental disorder that we will study using the ScreenChip system and we get to contribute to the development of a drug that may help children around the world.
Since NPC presents itself in early development, we will monitor the worms through early larval stages to try to mimic the early onset of NPC in human patients. Our first goal is to understand how clinical genetic variants affect the worm’s behavior.
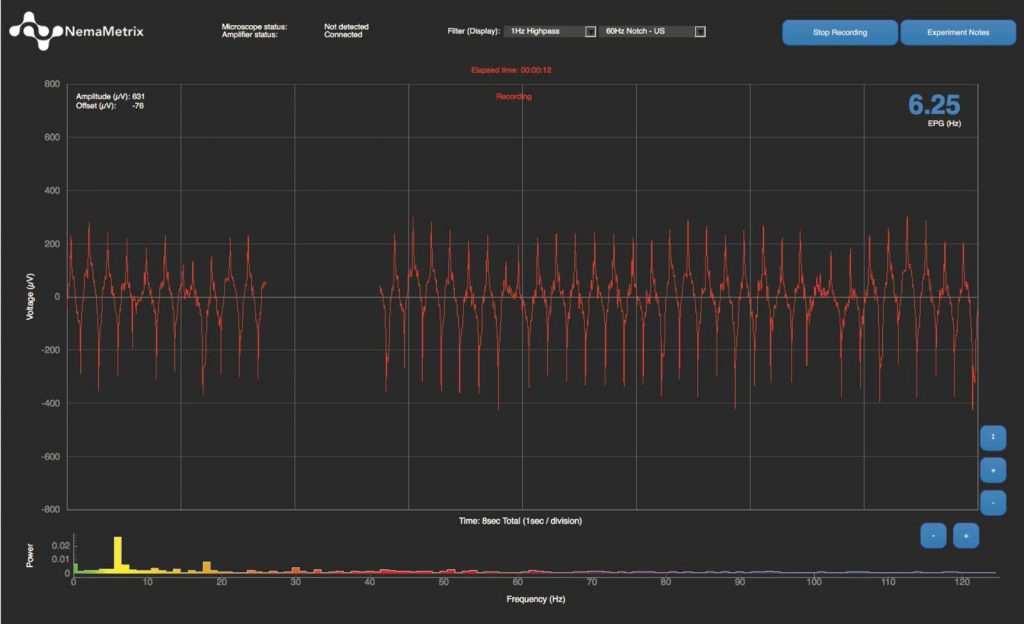
To do so, we will focus on C. elegans’ feeding behavior, which is a powerful readout of the worm’s health (e.g., Yu and Driscoll 2011). Measuring pharyngeal pumping in the first larval stage has proven extremely challenging thus far because the larvae are so tiny. We recently designed a chip (with a tiny channel – it’s only 10 µm tall!) that allows us to monitor the electric signal (or EPG) produced during feeding for this larval stage as we do for adult worms (see figure below). If we are able to establish a behavioral pattern in NPC larvae and adults, our goal is to explore the efficacy of Perlara’s new potential therapeutics.
The above image (left) shows a young adult worm in a standard size chip with 40 µm channel height at the narrow-most point; for comparison, the channel height for the L1 chip is 10 µm! We are able to monitor the electric signal as the worm’s pharynx (arrowhead) contracts and relaxes via two electrodes (indicated with asterisks). The right panel shows the corresponding digital readout of this electric signal.
Before undertaking this research, however, it was necessary for us to bolster our overall understanding of NPC. Here are the basics:
The lysosome is an organelle that digests, sorts and recycles molecules and fragments that are destined to be used by other parts of the cell. Lysosomal storage disorders (or LSDs) constitute a group of inherited disorders where lysosomal function is compromised, which leads to an accumulation of unwanted material within the lysosome as well as an overall reduction of lysosome-mediated intracellular transport. These diseases present themselves in children and often result in death at early ages. Niemann-Pick Type C is one of approximately 50 known LSDs.
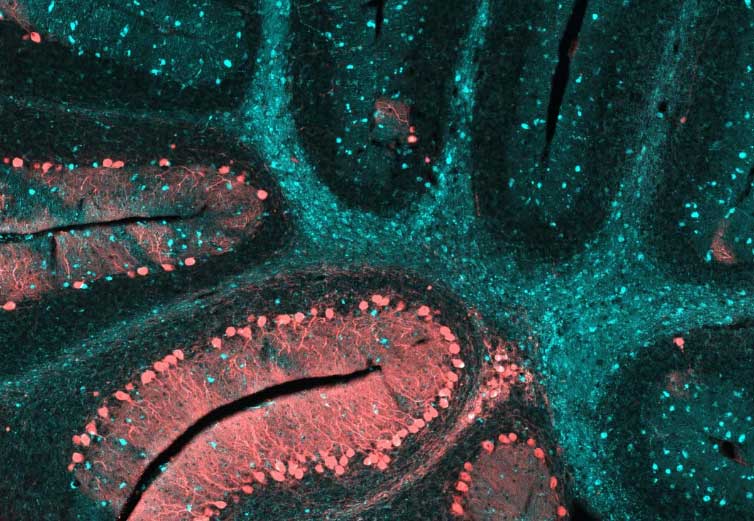
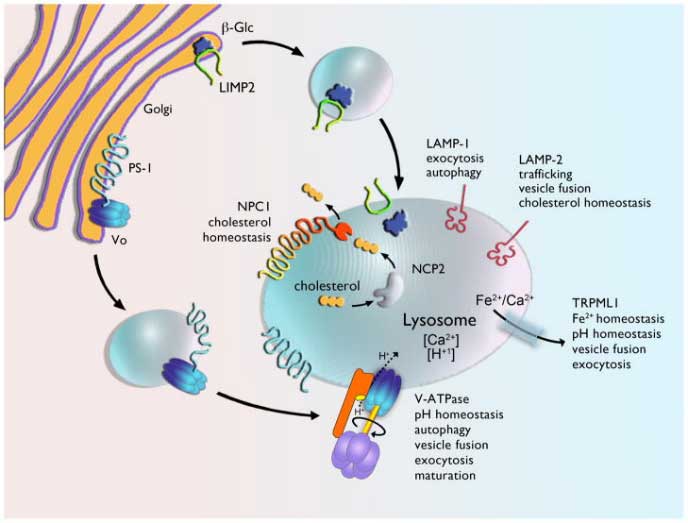
The left diagram is from Schultz et al., 2011, and displays organization and function of membrane proteins necessary for lysosomal function and cholesterol homeostasis (e.g., NPC1). The image on the right (from NIH Jan 2015 news release) shows the cerebellum of a brain affected by Niemann-Pick Type C; blue staining shows the dense pockets of lipid accumulations throughout the brain.
NPC is an autosomal recessive and often fatal neurodegenerative LSD caused by mutations to one of two genes: NPC1 and NPC2. We are focusing on NPC1, which encodes a membrane protein that regulates the transport of cholesterol (see Figure above, left panel) and accounts for most incidences of NPC. Defects in this gene lead to an accumulation of cholesterol in the lysosome as well as a deficit of cholesterol elsewhere in the cell (e.g., nucleus, endoplasmic reticulum). Treatments for NPC usually focus on treating its symptoms, which include liver failure, neurodegeneration, muscle degeneration, etc. Additional treatments and their market status are reviewed in Kirkegaard 2013.
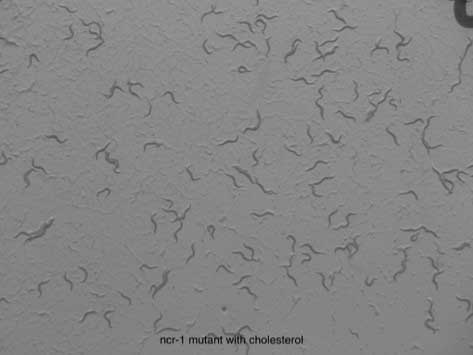
There are almost 58 C. elegans homologs of human genes associated with LSDs (deVoer et al. 2008). In 2000, Sym et al. discovered two NPC-like genes in C. elegans and created worm models bearing deletions to these genes, ncr-1 and ncr-2. C. elegans ncr-1 mutants exhibit similar abnormalities to those observed in the human system, including mortality at premature larval stages and defective cholesterol trafficking. (Relevant phenotypes have also been observed for ncr-2 mutants, but they are less dramatic than in ncr-1.) Other phenotypes observed in these NPC models include slowed growth, smaller brood sizes and proclivity to enter dormant (‘dauer’) stage (see Perlara blog posts). Furthermore, NPC worms display sensitivity to a lack of cholesterol, and phenotypes that may be rescued when mutants are supplanted with cholesterol. In a study in 2004 by Li et al., fluorescent reporter genes were used to illustrate the expression of ncr-1 in many cell types as well as its function of ncr-1 in cholesterol trafficking.
The above figures (from a Perlara blog post) illustrate the necessity of cholesterol in C. elegans development (worms at left grown with cholesterol; those at right were deprived of cholesterol).
Regardless of a sometimes negative connotation for its link to heart disease, cholesterol is very important for and synthesized in animal cells – it is essential for cell membrane structure and movement. C. elegans also need cholesterol, particularly for development into adulthood (Matyash et al., 2004), however, they do not synthesize it; instead, they must acquire it from their surroundings or food. Because the effects of NPC may be magnified in the absence of cholesterol, our experiments will include treatments for worms grown on plates with and without cholesterol.
What do we expect to find?
Studies of feeding behavior in C. elegans suggest a link to overall worm health. The pharynx itself is a neuromuscular organ, the function of which has been shown to be reduced or altered in other neurodegenerative disease models (e.g., Alzheimer’s, Weeks et al., 2016; Parkinson’s disease, Cooper et al 2015). In addition to neurodegeneration, NPC patients often experience muscle degeneration and reduced motor control, leading us to suspect that pharyngeal pumping will be affected. As a loss of appetite is observed in some NPC patients, feeding (i.e., pharyngeal pumping) may also be impacted directly. Stay tuned!
Read Part II: New phenotype was discovered for NPC disease
Unlock the potential of your research with our precise and efficient C. elegans genome-editing services – Contact us now!