Parkinson’s disease (PD) moves slowly, taking decades to develop, and impacting patients over decades of their lives. Considering that this debilitating condition affects over 10 million people worldwide, it represents a substantial social and economic burden. Zebrafish have emerged as a key model for understanding the disease, but its advantages for therapeutic discovery remain under utilized. In this post, we discuss how zebrafish provide an unparalleled model with still largely untapped potential for addressing Parkinson’s and other neurodegenerative disease.
Key Points
- Accurately modeling chronic degenerative diseases that develop in humans is difficult in any animal, but zebrafish offers some solutions.
- The diverse genetic factors that lead to Parkinson’s underscores the need for precise, accurate, and fast animal models such as zebrafish.
- Toxins such as MPTP can be used to induce acute models of PD for testing drugs relevant to progressed stages of the disease.
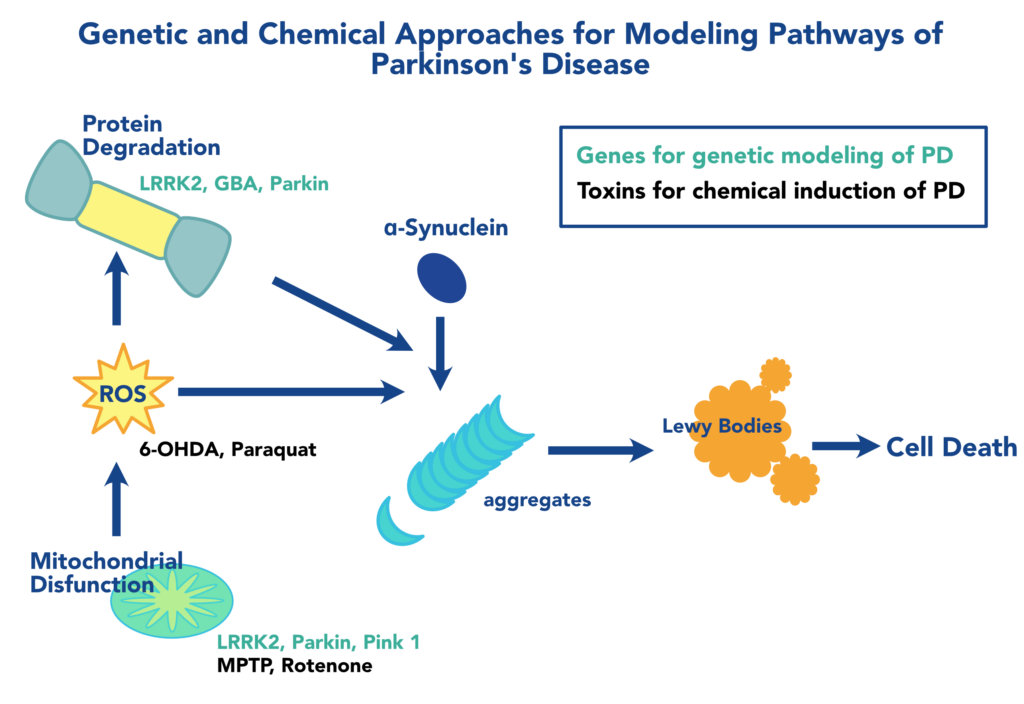
How do you study a disease that progresses over decades?
For a neurodegenerative disorder like Parkinson’s Disease (PD) that can take decades to develop in humans, how does one model the disease to discover new treatments on a timeline relevant to existing patients?
This is where the zebrafish shines.
PD has not been observed in any animals outside humans, and no animal model exists that replicates the complex and prolonged unfolding of the disease. Therefore, the accepted and effective approach has been to focus on the underlying molecular, cellular, and neurological mechanisms of PD. Within this mechanistic framework, there is no other model that makes the full spectrum of PD pathophysiology as visible as zebrafish. This owes to four key advantages:
- The transparency and external development of zebrafish larvae provides a window through which cellular processes and neuroanatomy can be observed using non-invasive procedures such as fluorescence microscopy.
- Abundant external broods can be genetically manipulated using morpholinos or crispant reagents without investing in costly genetic engineering or compromising precious parental lines.
- Each breeding pair can produce hundreds of embryos providing high replicate numbers and statistical confidence.
- High throughput screening methodologies and instruments have been established for performing drug screens using phenotypic or behavioral readouts.
Most importantly, zebrafish biologically capture relevant anatomical, pathophysiological, and behavioral aspects of the disease. They also respond congruently to drugs and perturbations that either induce or ameliorate symptoms of the PD. Although the differences between humans and zebrafish neuroanatomy are profound, researchers have extensively mapped the developing zebrafish nervous system and identified the regions of the brain and CNS functionally and pathologically homologous to those in the human brain impacted by PD. Specifically useful for PD research are the similarities within the human and zebrafish dopaminergic system, where the pathways leading to PD most prominently manifest.
Human genes involved in the pathways leading to PD have orthologs in zebrafish that, when similarly mutated, elicit phenotypes emblematic of Parkinson symptoms. Additionally, toxins that induce Parkinson’s like states in mammals do likewise in zebrafish. Having a wide selection of both genetic and toxin-induced models available is another aspect that has empowered researchers to pursue the mechanistic drivers of PD. Here, we discuss some of the more prominent genetic and chemical models of PD.
Genetic Models of Parkinson’s
PD can result from either genetic or environmental factors. The genetic factors can involve one or more of several genes and can take on both autosomal recessive and autosomal dominant forms. Depending on which gene is mutated and how it’s function is impacted, patients exhibit a range of physiological and neurological symptoms. Since many different pathways can be disrupted in a way that lead to PD, it is important to develop precise and accurate models for target identification and drug discovery.
Synuclein
The canonical pathophysiology of PD results from the death of dopaminergic (DA) neurons due protein aggregates known as Lewy Bodies. α-Synuclein (α-syn) is the central player, forming the core component of Lewy bodies when misfolded α-syn monomers nucleate self-propagating aggregates. Zebrafish express three genes that encode the β-, γ1 , and γ2 forms of synuclein. Although ZF does not possess a direct α-syn ortholog, expression patterns in the brain, developmental regulation, and PD-related knockdown phenotypes suggest that γ1 syn is a functional homolog of α-syn. This is supported by the phenotypic rescue by human α-syn. Several clients have taken advantage of our genome editing technologies to create precise models of Synuclein variants in zebrafish for modeling Parkinson’s.
LRRK2, GBA, Parkin, Pink-1, DJ1
Mutations in several other genes with orthologs in zebrafish have been known to cause PD. These are involved in overlapping pathways of protein homeostasis, reactive oxygen species, and mitochondrial maintenance that feed into the protein folding defects that lead to α-syn aggregation, with some notable exceptions. Zebrafish express orthologs of these genes that have been used to better understand PD and the unique pathology of different patient variants.
Genetic studies of Parkinson’s in ZF have greatly relied on either genetic knock-outs, knockdowns using Morpholinos, or altered gene expression levels. This often falls short of capturing the pathophysiology of a disease where point mutations might be at the root of protein misfolding and aggregation. There remains great opportunity for discovery in using genome editing to precisely model specific pathologic variants associated with PD. Numerous clients have leveraged our genome editing expertise to construct precise and accurate genetic models of Parkinson’s and other neurological diseases.
Our Parkinson's Modeling Platform
Chemical Models of Parkinson’s
Chemical models offer a straightforward, generalized, and flexible alternative to genetic models of PD. Genetic models are ideal for investigating the mechanisms underlying the pathology of specific variants, or for discovery of treatments for specific forms of the disease. However, they face the hurdles of not only investing the time and resources to construct the model, but also testing whether any new model accurately reflects the disease state. Addtionally, effects of a mutation on early development might confound investigation of relevant behavioral phenotypes at later stages or adults. Four common chemicals used to model PD in zebrafish are 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), 6-hydroxydopamine (6-OHDA), paraquat, and rotenone]. These chemicals have caused decreases in DA neurons and effects on locomotion and behavior of larval zebrafish.
MPTP and 6-OHDA
In the early 1980’s, researchers investigated several cases in which illicit drug users had mysteriously developed PD. The culprit was a contaminant, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), which is now perhaps the most commonly-used chemical for inducing PD in animal models, including zebrafish. The utility of MPTP comes from its selective killing of dopaminergic (DA) neurons, through enzymatic conversion to MPP+ which is imported through the dopamine receptor (DAT). MPTP exposure led to decreased DA neurons in the ventral diencephalon and decreased swimming speed in larval zebrafish (Kalyn et al., 2020; Wasel and Freeman, 2020). 6-hydroxydopamine (6-OHDA) has also been used in zebrafish studies. It likewise selectively enters DA neurons through the DAT, but kills cells through a different mechanism involving reactive oxygen species.
Paraquat, Rotenone, and Environmental Toxins.
Environmental toxins, including pesticides and air pollution have been associated with increase risk of developing PD. Paraquat is a pesticide structurally similar to MPTP, but was found to act through an entirely different mechanism. Unlike MPTP, Paraquat is not imported by DAT, but is a redox-active molecule that increases oxidated stress in DA neurons. Rotenone is a naturally-occuring compound that has been used as a broad-spectrum pesticide. It is an inhibitor of mitochondrial complex I like Paraquat, increases oxidative stress. Although such toxins have been associated with PD, their role in research may be more geared towards determining the causality of these associations, than creating models for therapeutic discovery.
Neurotoxin exposure remains a straightforward method of modeling PD. The caveat of taking temporal control of the induction of PD, however, is that the timing of exposure must be carefully coordinated with developmental stage to accurately capture the phenotypes relevant to human pathology. Our team of scientists collectively have decades of experience in zebrafish developmental biology and neuroscience, as well as the resources of internationally-recognized facilities. They have helped numerous clients execute valid and reproducible assays to obtain preclinical data.
Complementary Approaches to Obtain the Right Insights.
The availability in zebrafish of both genetic and toxin-induced models of PD enables researchers to fine-tune their approach to relevant facets of the disease. With more options, however, comes more considerations. While neurotoxins can acutely induce some hallmarks of PD in zebrafish, genetic approaches may be better suited to investigating the molecular mechanisms through with genetic variants cause PD. However, new genetic models may require additional testing and time to validate, so chemical models of PD in zebrafish still have excellent value as a tool for drug discovery and drug repurposing for PD treatment. .
At InVivo Biosystems we have helped numerous clients construct genetic models for advancing Parkinson’s research. Additionally, we have extensive experience with the nuances of toxin-induced models and have the capabilities for high-througput behavioral phenotyping. We recognize the extraordinary power of the zebrafish model and are pressing forward in developing the tools to empower researchers in addressing neurodegenerative disorders.
References
Barnhill, L.M., Murata, H., Bronstein, J.M., 2020. Studying the pathophysiology of Parkinson’s disease using zebrafish. Biomedicines 8, 197. https://doi.org/10.3390/biomedicines8070197
Feng, C.-W., Wen, Z.-H., Huang, S.-Y., Hung, H.-C., Chen, C.-H., Yang, S.-N., Chen, N.-F., Wang, H.-M., Hsiao, C.-D., Chen, W.-F., 2014. Effects of 6-hydroxydopamine exposure on motor activity and biochemical expression in zebrafish (Danio rerio) larvae. Zebrafish 11, 227–239. https://doi.org/10.1089/zeb.2013.0950
Kalyn, M., Hua, K., Mohd Noor, S., Wong, C.E.D., Ekker, M., 2020. Comprehensive analysis of neurotoxin-induced ablation of dopaminergic neurons in zebrafish larvae. Biomedicines 8, 1. https://doi.org/10.3390/biomedicines8010001
Keow, J., 2016. Physiological and behavioral changes in a rotenone model of dopamine neurotoxicity and neurodegeneration in zebrafish (Thesis). Université d’Ottawa / University of Ottawa. https://doi.org/10.20381/ruor-339
Nishimura, Y., Inoue, A., Sasagawa, S., Koiwa, J., Kawaguchi, K., Kawase, R., Maruyama, T., Kim, S., Tanaka, T., 2016. Using zebrafish in systems toxicology for developmental toxicity testing. Congenit. Anom. 56, 18–27. https://doi.org/10.1111/cga.12142
Razali, K., Othman, N., Mohd Nasir, M.H., Doolaanea, A.A., Kumar, J., Ibrahim, W.N., Mohamed Ibrahim, N., Mohamed, W.M.Y., 2021. The promise of the zebrafish model for Parkinson’s disease: today’s science and tomorrow’s treatment. Front. Genet. 12, 553. https://doi.org/10.3389/fgene.2021.655550
Redgrave, P., Rodriguez, M., Smith, Y., Rodriguez-Oroz, M.C., Lehericy, S., Bergman, H., Agid, Y., DeLong, M.R., Obeso, J.A., 2010. Goal-directed and habitual control in the basal ganglia: implications for Parkinson’s disease. Nat. Rev. Neurosci. 11, 760–772. https://doi.org/10.1038/nrn2915
Stewart, A.M., Braubach, O., Spitsbergen, J., Gerlai, R., Kalueff, A.V., 2014. Zebrafish models for translational neuroscience research: from tank to bedside. Trends Neurosci. 37, 264–278. https://doi.org/10.1016/j.tins.2014.02.011
Wasel, O., Freeman, J.L., 2020. Chemical and genetic zebrafish models to define mechanisms of and treatments for dopaminergic neurodegeneration. Int. J. Mol. Sci. 21, 5981. https://doi.org/10.3390/ijms21175981




