Summary
Inflammation’s role in diseases has become increasingly apparent, with inflammatory responses being linked to diseases as common as arthritis, and as debilitating as Parkinson’s. A promising way to counteract inflammation is through natural compounds – currently these compounds are being tested on the well-suited alternative animal model, zebrafish. In this article we discuss the aspects of the zebrafish which make it an ideal model for inflammation research.
Whether or not you are always aware of it, we have all experienced inflammation at various points in our lives. There are two types of inflammation – acute and chronic. The acute inflammatory response our bodies produce is necessary for defense against pathogens and for tissue repair after injury, even for injuries as small as paper cuts! However, inflammatory responses can also harm tissues, and chronic inflammation has been associated with numerous diseases, including Type 2 diabetes, Parkinson’s disease, inflammatory bowel disease, arthritis, heart disease, and certain cancers.1 There are a number of approved drugs already on the market, but current drugs to control inflammation, such as glucocorticoid steroids, can have multi-system effects and lead to undesired outcomes including hypertension, osteoporosis, diabetes, and infections.2 In an order to minimize undesired outcomes and still provide targeted therapies for inflammation-associated diseases, many researchers have started to turn towards natural compounds with anti-inflammatory effects.
Natural compounds to treat inflammation are nothing new. A number of herbs from traditional Chinese medicine have already shown anti-inflammatory activity in cell and animal models.3 While natural compounds are used worldwide to treat illnesses and pain, it is necessary to question whether they confer any benefits to the user (i.e. do they actually work?) Every country has different rules and regulations around monitoring nutraceutical compounds, but for the purpose of this article we will be focusing on the United States. The USA’s nutraceutical market is regulated by two separate agencies, the FDA and FTC. Both the FDA and the FTC require scientific evidence for dietary supplements’ safety and efficacy, which is where a cheap, fast, and easily manipulated alternative animal model like the zebrafish is so beneficial.
To read more about the regulations surrounding the dietary supplement industry in the USA and abroad read our recent article: A Closer Look at Nutraceutical Regulations: How Does the USA Compare?
Zebrafish are an excellent model for testing anti-inflammatory compounds for both efficacy determination and mechanism characterization. Already well-known as a model organism in the fields of developmental biology, neuroscience, and toxicology, the zebrafish has also been used as a model for inflammation and immune-modulating drug discovery. Due to their high fecundity, rapid growth, and relatively inexpensive husbandry requirements, it is faster and less expensive to screen compounds in zebrafish, compared to mammalian models. These attributes, along with being able to house larval stages in multi-well plates, makes larval zebrafish excellent candidates for rapidly screening natural compounds.
As a vertebrate animal, the zebrafish shares many of the same organs and immune functions as the human immune system, including innate immune responses involving macrophages4 and neutrophils,5 the complement system,6 homologs to human cytokines,7 and mucosal immune responses.8 At 4-6 weeks post-fertilization, zebrafish have functional adaptive immunity,9 including antibodies and T-lymphocytes that recognize specific pathogens. Zebrafish have the additional benefits of optical transparency during the first 2 weeks of life, which is especially useful in visualization. For example, transgenic lines that express cell-specific fluorescent markers have been created to visualize immune cell migration in live fish, giving a rapid readout of the inflammatory response.
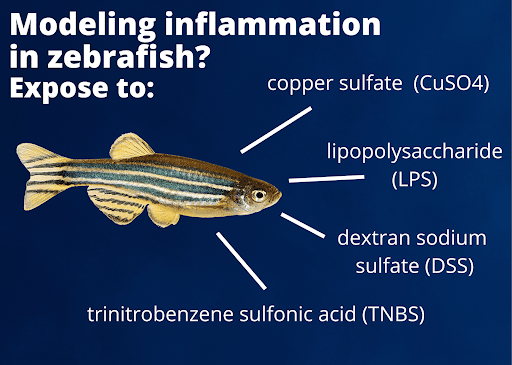
Figure 1. Commonly-used methods to induce inflammation in zebrafish larvae.
There are multiple options for modeling inflammation in zebrafish. Copper sulfate (CuSO4), lipopolysaccharide (LPS), dextran sodium sulfate (DSS), or trinitrobenzene sulfonic acid (TNBS) are all chemicals used to induce inflammation by bath exposure in larvae,10 making treatment of large numbers of fish an efficient and cost-effective option [Figure 1]. While these chemicals can produce inflammation throughout the body and are not always site-specific, TNBS-caused inflammation is somewhat more targeted to the intestine.11 Inflammation can also be modeled in larvae by wounding the animal at specific body sites (usually amputating the caudal fin, which will regenerate),12 which may be more appropriate for testing natural products that will be directed at skin or wound healing.
Other assays to investigate the mechanisms of action of anti-inflammatory compounds include measuring the expression of inflammatory markers (Table 1) by RT-qPCR or RNA-seq. This approach has already been used to test traditional medicine compounds in zebrafish for use as anti-inflammatory treatments. For example, treatment with Gardenia jasminoides extract (a traditional Chinese medical herb) decreased macrophage migration and pro-inflammatory cytokine expression.13 Another study examined stem and leaf extracts from chrysanthemum (used in traditional Chinese medicine), which decreased expression of the inflammation markers IL-1β, IL-8, and MMP9 in a zebrafish model of DSS-induced inflammatory bowel disease (IBD).14
With cases of IBD increasing worldwide, the discovery of better therapeutics could be life-changing for millions of people, particularly because the current course of treatment usually relies onmedicines such as corticosteroids and other immunosuppressive drugs which can be expensive and have long-lasting side effects.15

Table 1. Commonly-measured inflammation markers in zebrafish
With the potential to be so impactful on human health and disease treatment, it is exciting to see the rise of zebrafish modeling of inflammation. Here at InVivo Biosystems, we use CRISPR knock-outs, knock-ins, and the Tol2 transposon system to create zebrafish models for a range of human diseases and drug discovery trials. We are proud to be part of the researchers that are using cutting-edge technology to advance our understanding of and ability to treat inflammation.
Ready to reshape the landscape of drug discovery and development? Contact us now to explore how our comprehensive services can accelerate the development of groundbreaking therapies.
References
- Inflammation. National Institute of Environmental Health Sciences https://www.niehs.nih.gov/health/topics/conditions/inflammation/index.cfm (2021).
- Harris, E., Tiganescu, A., Tubeuf, S. & Mackie, S. L. The prediction and monitoring of toxicity associated with long-term systemic glucocorticoid therapy. Curr. Rheumatol. Rep. 17, 38 (2015).
- Pan, M.-H., Chiou, Y.-S., Tsai, M.-L. & Ho, C.-T. Anti-inflammatory activity of traditional Chinese medicinal herbs. J. Tradit. Complement. Med. 1, 8–24 (2011).
- Mathias, J. R. et al. Characterization of zebrafish larval inflammatory macrophages. Dev. Comp. Immunol. 33, 1212–1217 (2009).
- Renshaw, S. A. et al. A transgenic zebrafish model of neutrophilic inflammation. Blood 108, 3976–3978 (2006).
- Boshra, H., Li, J. & Sunyer, J. O. Recent advances on the complement system of teleost fish. Fish Shellfish Immunol. 20, 239–62 (2006).
- Zou, J. & Secombes, C. J. The function of fish cytokines. Biology 5, 23 (2016).
- Salinas, I. The mucosal immune system of teleost fish. Biology 4, 525–539 (2015).
- Lam, S. H., Chua, H. L., Gong, Z., Lam, T. J. & Sin, Y. M. Development and maturation of the immune system in zebrafish, Danio rerio: a gene expression profiling, in situ hybridization and immunological study. Dev. Comp. Immunol. 28, 9–28 (2004).
- Xie, Y., Meijer, A. H. & Schaaf, M. J. M. Modeling inflammation in zebrafish for the development of anti-inflammatory drugs. Front. Cell Dev. Biol. 8, (2021).
- Oehlers, S. H. et al. A chemical enterocolitis model in zebrafish larvae that is dependent on microbiota and responsive to pharmacological agents. Dev. Dyn. 240, 288–298 (2011).
- Wang, X. et al. Inhibitors of neutrophil recruitment identified using transgenic zebrafish to screen a natural product library. Dis. Model. Mech. 7, 163–169 (2014).
- Chen, J. et al. Anti‑inflammatory activities of Gardenia jasminoides extracts in retinal pigment epithelial cells and zebrafish embryos. Exp. Ther. Med. 22, 1–9 (2021).
- Li, Y. et al. Evaluation of anti-inflammatory and antioxidant effects of chrysanthemum stem and leaf extract on zebrafish inflammatory bowel disease model. Molecules 27, 2114 (2022).
- Nguyen, T. H. et al. Anti–inflammatory and antioxidant properties of the ethanol extract of Clerodendrum cyrtophyllum Turcz in copper sulfate-induced inflammation in zebrafish. Antioxidants 9, 192 (2020).




