Summary:
Drug repurposing takes previously known compounds and drugs, and applies them to current health problems. Drug repurposing is particularly beneficial for finding rare disease therapies because it offers an accelerated and economical alternative to traditional drug discovery, a process which is often deemed too costly and time consuming for the small population pools affected by rare diseases. Drug repurposing also broadens treatment options for a myriad of health concerns. This article will discuss drug repurposing, its current complications, and how despite these complications the drug repurposing pipeline is an underutilized tool that is able to provide new treatment options as well as insights that further traditional drug discovery.
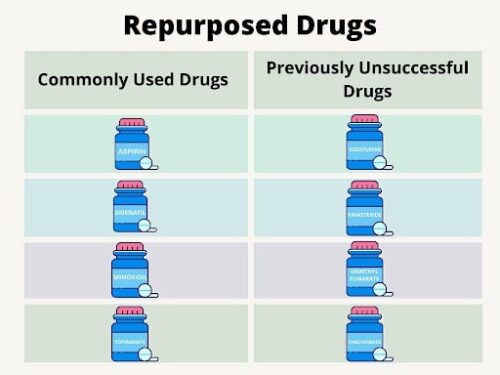
Nearly 10,000 drug compounds are currently approved for clinical use in the USA and Europe, but this amount represents only 15% of total drugs that reached the clinical trial phase. The remaining 85% of drugs fail to gain approval at various stages of clinical trials (Corsello et al., 2017). Ignoring these drugs represents a loss in potential treatment options. This is where drug repurposing comes into play. While drug repurposing can involve commonly used drugs, one of the most common avenues for drug repurposing is through revitalizing old, unused compounds, and finding new and safe uses for them. Since these compounds have already gone through a significant portion of the drug development pipeline, their approval timeline is significantly shorter and less difficult than de novo discovery. Thus, drug repurposing benefits anyone looking for more treatment options as there are thousands of compounds available for continued testing and new use.
Historical Relevance
Drug repurposing takes previously known compounds and drugs, and applies them to current health problems. Drug repurposing is particularly beneficial for finding rare disease therapies because it offers an accelerated and economical alternative to traditional drug discovery, a process which is often deemed too costly and time consuming for the small population pools affected by rare diseases. Drug repurposing also broadens treatment options for a myriad of health concerns. This article will discuss drug repurposing, its current complications, and how despite these complications the drug repurposing pipeline is an underutilized tool that is able to provide new treatment options as well as insights that further traditional drug discovery.
Although drug repurposing is not a new approach, its methods have evolved since its origin. Drug repurposing currently utilizes semi-systematic methods, but its beginnings are rooted in serendipity. An early example of drug repurposing is the case of the drug thalidomide – taken off the market for its original application over health concerns, it became a success story when given as a final effort to minimize leprosy-related pain.
Marketed by German company Chemi Grunenthal, thalidomide was developed as a treatment for morning sickness during pregnancy; however, the company solely analyzed the effects on the mothers, failing to consider the effects this drug could have on the babies. Consequently, while thalidomide did reduce morning sickness in pregnant women, their children were born with severe congenital defects. After only five years on the market, in 1961 the drug was recalled due to the detrimental effects it had on the children (Pushpakom, 2019).
Although thalidomide was commercially banned, it remained available in some countries, such as Israel. In 1964, at Hadassah University in Jerusalem, Dr. Jacob Sheskin prescribed thalidomide to his patient suffering from erythema nodosum leprosum (ENL), or leprosy, as a last resort sedative for his extreme pain. Within 48 hours, Dr. Sheskin noticed “unexpected and dramatic resolution of the patient’s lepra skin eruption” (Rehman et al., 2011). Sheskin reproduced and reported these results in 1965, and in 1971 the World Health Organization (WHO) found efficacy of thalidomide for ENL following randomized trials (Rehman et al., 2011). In the 1970s, the FDA supported testing thalidomide’s use for various inflammatory conditions. Subsequent clinical trials found thalidomide to have “anti-emetic, sedative, and analgesic properties,” allowing for its repurposing in a palliative care setting as well. (Rehman et al., 2011).
Thalidomide is a concrete example of how a drug can be repurposed from its original intentions and useful if used in the right conditions, and can improve treatment options for conditions that may already have therapeutic options.
Applications of Drug Repurposing
Drug repurposing has several applications : rare disease therapy, precision medicine, and treatment with reduced cost and time. Traditional drug discovery often overlooks rare disease since those therapies cater to such a small group of individuals. However, drug repurposing removes the inevitable commercial burden of rare disease therapy discovery by reducing financial burdens. This is made feasible by bypassing molecule discovery, in vivo testing, drug creation, and potentially even Phase I clinical trials. Through drug repurposing, pharmaceutical companies are able to develop rare disease therapies without unsustainable revenue losses. The accelerated timeline of drug repurposing is also vital for rare disease patients, who often require immediate care and cannot afford to wait 10-15 years for a solution (Roessler, 2021).
Drug repurposing is an extremely useful tool for precision medicine as well, by providing treatments on a small, case by case basis based on a patient’s diagnosis, genetic makeup, health history, and symptoms. For example, many cancers “comprise a spectrum of effects”, which means a patient may have a unique array of multiple symptoms and by tailoring a patient’s treatment to their genome they receive a treatment that is designed for them.
In addition to creating more targeted therapies and reducing the time to market, drug repurposing also aids rare and neglected diseases by lowering the financial barrier.
Where Drug Repurposing is Today
Today, drug repurposing functions as an expedited method for compounds to gain FDA approval for new and alternative uses to previously underutilized compounds. The drug repurposing process begins with in vivo trials of the known drug, or even Phase II clinical trials if “the required strength for the new indication is equal or lower than the one used for the original indication” (Talevi & Bellera, 2020). This head start allows researchers to begin the drug approval process with clinical trials. They must complete the remaining phases in order to obtain the green light from the FDA.
While many repurposed drugs were originally found through chance, today researchers work to systemize the process to rapidly find solutions. The path to repurposing drugs now begins with scanning libraries and databases of previously approved drugs and compounds. Compounds showing positive indications for their disease target, such as blocking the pathway for disease development, are selected. Once selected, compounds are scanned again to determine their specific indications for the disease in question, such as reduction of tumor size or anti-inflammatory qualities (Gil & Martinez, 2021). Drug repurposing does not solely find treatments for known diseases, but also looks for compounds with phenotypic or mechanistic effects on specific targets. This gives compounds polypharmacy, or the ability to treat multiple diseases, allowing for current and new applications. (Talevi & Bellera, 2020).
Concluding Remarks
Discovered through serendipity, researchers today are working to create a systemic approach to drug repurposing in order to develop a straightforward approval pathway. The accelerated process significantly reduces financial burden on drug developers and researchers, allowing them to explore a wider array of diseases, including those that are currently untreated. This creates hope for patients living with diseases that are deemed economically unfeasible to treat.
Furthermore, drug repurposing serves a greater benefit – providing databases mapping mechanisms and indicators which will show us new cellular pathways, aiding all forms of drug discovery (Gil & Martinez, 2021). This exponentially grows the ability of scientists to provide cures and treatment for vast arrays of health concerns. Drug repurposing creates a larger opportunity to find solutions to any condition, furthering the healthcare industry and improving everyone’s ability to receive care.
References
- Corsello, S., Bittker, J., Liu, Z. et al. The Drug Repurposing Hub: a next-generation drug library and information resource. Nat Med 23, 405–408 (2017). https://doi.org/10.1038/nm.4306
- Pushpakom, S., Iorio, F., Eyers, P. A., Escott, K. J., Hopper, S., Wells, A., Doig, A., Guilliams, T., Latimer, J., McNamee, C., Norris, A., Sanseau, P., Cavalla, D., & Pirmohamed, M. (2019). Drug repurposing: progress, challenges and recommendations. Nature reviews. Drug discovery, 18(1), 41–58. https://doi.org/10.1038/nrd.2018.168
- Roessler, H. I., Knoers, N., van Haelst, M. M., & van Haaften, G. (2021). Drug Repurposing for Rare Diseases. Trends in pharmacological sciences, 42(4), 255–267. https://doi.org/10.1016/j.tips.2021.01.003
- Gil, C., & Martinez, A. (2021). Is drug repurposing really the future of drug discovery or is new innovation truly the way forward?. Expert opinion on drug discovery, 16(8), 829–831. https://doi.org/10.1080/17460441.2021.1912733
- Talevi, A., & Bellera, C. L. (2020). Challenges and opportunities with drug repurposing: finding strategies to find alternative uses of therapeutics. Expert opinion on drug discovery, 15(4), 397–401. https://doi.org/10.1080/17460441.2020.1704729