Summary
Getting a drug to market is notoriously difficult, taking 7-10 years and costing hundreds of millions of dollars. This may make you wonder whether your drug is eligible for an expedited track. In this article we will discuss the current ways of expediting drugs, and which is best for your research.
The FDA’s expedited drug program was started to help address the difficulty of getting drugs for rare diseases to market. Rare diseases often have small and diverse patient populations, which makes it difficult for a rare disease therapy to meet the milestones that are typically required for FDA approval. Today, there are four distinct ways researchers can rapidly make their drug available: Fast Track, Breakthrough Therapy, Accelerated Approval, and Priority Review. These approaches are still used to facilitate rare disease therapies, however, they have expanded to include other ‘serious diseases’ as well, such as tropical diseases, heart failure, and cancers.
The FDA’s expedited drug tracks help bring a drug to market faster, however, there is a barrier in developing drugs for rare diseases due to the cost. In addition to the cost, the small market size of rare disease drugs may disincentivize pharmaceutical companies from pursuing these drugs as the ROI may seem non-existent (Grabowski & Moe, 2006). In response, the FDA created priority review vouchers to make the drug pipeline faster by reducing the review time of voucher drugs to six-months. The three available vouchers cover certain tropical diseases, rare pediatric diseases, and medical countermeasures to material threats such as a biological, chemical, or nuclear agent.
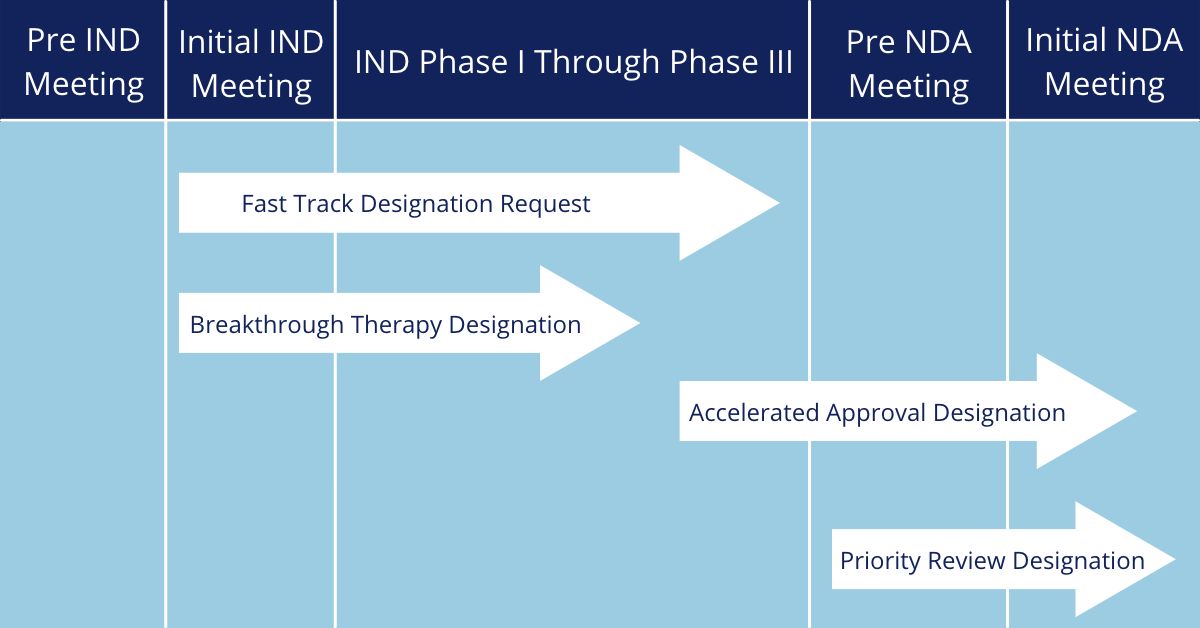
Figure 1. Development timelines and when each can be applied for for the different regulatory expedited designation programs
Expedited Programs:
- Fast Track – if you don’t have clinical data but want your drug application reviewed in steps instead of having to wait until the entire application is finalized
- Breakthrough Therapy – if you have early clinical data and want to skip steps of the standard review
- Accelerated Approval – if you want your drug approved before conclusive clinical data can be collected
- Priority Review – if you want your drug reviewed sooner
Priority Review Vouchers
- Tropical Disease, Rare Pediatric Disease, Medical Countermeasures: if you have a drug that you want reviewed within 6 months without having to apply, fits more specific requirements than Priority Review Expedited Track, and are willing to pay the additional fee
Fast Track
Established a few years into the existence of the Expedited Programs, the Fast Track was born out of the “Administration Modernization Act of 1997.” This act intended to set the stage for FDA operations in the 21st Century – it recognized the complexities of the evolving drug pipeline, and in response, aimed to facilitate the development and expedite the review of new drugs. Drugs that are eligible for a Fast Track designation only need to show the potential to “treat serious conditions and fill an unmet medical need,” through preliminary data such as a nonclinical model, pharmacological data, or mechanistic rationale. Thus, a drug will be considered if no other treatment exists or offers a substantial benefit over existing treatment. According to the FDA, a serious condition is defined as: impacting survival, day-to-day functioning, “or the likelihood that the condition, if left untreated, will progress from a less severe condition to a more serious one.”For example, drugs treating AIDS, Alzheimer’s Disease, heart failure, cancer, epilepsy, depression and diabetes have all received Fast Track designation. Applicants are recommended to apply as early as Investigational New Drug (IND) application and prior to Biologics License Application (BLA) or New Drug Application (NDA) submission. If approved, drugmakers will need to meet frequently with the FDA to provide updates, however, this can be beneficial as the FDA may permit Fast Track drugs to have a rolling review. This means that the FDA will review sections of a NDA as they are completed rather than waiting until all aspects are finalized. A Fast Track drug can also receive Accelerated Approval and Priority Review, if it meets the criteria, which could further cut down on the drug approval timeline.
Breakthrough Therapy
The Breakthrough Therapy pathway was formed with the goal of expediting the development and review of new drugs whose preliminary clinical trials are extremely promising. The Breakthrough Therapy designation is a newer pathway, enacted in 2012 by the Food and Drug Administration Safety and Innovation Act (Darrow, Avorn & Kesselheim, 2014). This track was created after a series of workshops, hosted by Friends of Cancer Research, discussed the promising therapies for difficult to treat cancers. They found that even when therapies were showing efficacy, and were targeted for early cancer development, the traditional clinical development programs required subjects to be exposed to other ineffective or outdated therapies. Eligible drugs have clinical data that “demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints.” In this description, the FDA defines ‘substantial improvement’ as the magnitude of treatment effect (can include duration), and importance of outcome, and ‘clinically significant endpoints’ as endpoints that measure irreversible morbidity or mortality, or serious symptoms. These endpoints can also suggest effects, such as an improved safety profile, an effect on a surrogate endpoint or on a pharmacodynamic biomarker. A Breakthrough Application can be submitted as early as IND application (best submitted before the end-of-Phase II meeting), once submitted the FDA will review it and respond within 60 days. If approved, the Breakthrough track offers frequent meetings with FDA to gain guidance on the drug approval starting at Phase 1, and the chance to be considered for rolling review (may be able to skip portions of standard review thanks to early clinical data). Notably, a Breakthrough Therapy can also receive all Fast Track benefits.
Accelerated Approval
The first, and perhaps most well known, of the expedited programs is the Accelerated Approval track. It was created in 1992 in response to the AIDS epidemic and has had a substantial effect on many public health crises since then. The Accelerated Approval program allows the FDA to approve drugs whose clinical benefit may still take years to determine, to patients with serious conditions as quickly as possible. Good drug candidates for Accelerated Approval are drugs whose effects are intended to treat people over a long period of time. These drugs need to show an effect on a surrogate endpoint, a measure thought to predict clinical benefit, but is not itself a measure of clinical benefit (i.e. radiographic image, physical sign or other). Or, these drugs must show an effect on an intermediate clinical endpoint, a measure that suggests clinical benefit (i.e. irreversible morbidity and mortality). ‘Serious conditions’ which would be eligible for Accelerated Approval include, but are not limited to, diseases such as cancers and HIV. Drugmakers hoping to receive Accelerated Approval ought to discuss it with the FDA during the drug’s development, and, if approved, will continue to have close contact with the FDA as accelerated approval is only a stepping stone on the path to full FDA approval. As such, drugs that have gone down this track must undergo clinical trials after approval in order to garner full FDA approval. Notably, until the drug is fully approved it must be clearly labelled as an ‘accelerated approval’ product. Furthermore, if the drug fails to show long-term safety and efficacy FDA will withdraw its approval entirely.
Priority Review
The Priority Review pathway aims to give extra attention to drugs with strong potential to improve treatment (safety or effectiveness) of a serious disease over existing therapies. The Priority Review pathway stems from the standard review process being overwhelmed – to help ease the application process, the Prescription Drug User Fee Act was passed in 1992, which set guidelines for drug review timelines and created the current two-tiered system. Unlike Fast Track, Breakthrough Therapy, and Accelerated Approval, a drug can apply for this pathway later in the application process. In addition to later application, this pathway can also be coupled with any of the other expedited programs. Drugs can be considered for Priority Review if they have clinical trial data that shows increased effectiveness in treatment, prevention, or diagnosis of a disease, a reduction in drug reaction, or benefit in a new subpopulation. Examples of diseases that Priority Review drugs have targeted include cancer, AIDS, Alzheimer’s, heart failure, Hepatitis C, and diabetes. Approval of Priority Review designation means that the FDA will review the drug within 6 months rather than the typical 10 months, cutting the timeline for a drug’s review process nearly in half.
Priority Review Vouchers
In addition to having various expedited tracks, the FDA offers three vouchers that a company may also choose to acquire. The vouchers include a voucher for certain tropical diseases, a voucher for certain rare pediatric diseases, and a voucher for drugs that act as a countermeasure to material threat. The goal of Priority Review Vouchers is to incentivize the development of new drug ingredients (not new innovations), by reducing the length of the approval pipeline these drugs must go through. While these vouchers may initially seem identical to the expedited tracks, they differ from the expedited tracks in that they can be sold, or ‘transferred’ to another manufacturer, who in turn, can apply the voucher to a drug product of their own making. The FDA does specify a list of diseases that qualify, but they also note that “any other infectious disease for which there is no significant market in developed nations and that disproportionately affects poor and marginalized populations” would be considered. To see the full list go to the FDA’s website. Qualifying drugs should meet all the criteria of the expedited program’s non-specific, Priority Review (at least one clinical investigation), and cannot contain any active ingredients that are used in a previously approved application. The main difference between the expedited program’s Priority Review track, and the Priority Review vouchers is that the vouchers can be applied to whichever drug the applicant wants, whereas, the FDA must approve the drug for an expedited track. However, this does not mean that voucher drugs are more likely to make it to market, just that the voucher will, “accelerate the approval of a good drug to market, but it will not save a bad drug from being rejected” (Mezher, Brennan & Gaffney, 2020). All drugs submitted using a voucher will be reviewed by the FDA within six months (~90% of drugs, with or without the voucher, are reviewed within this timeframe). Notably, the voucher programs have seen legislative change in the last decade – in 2012 the Rare Pediatric Voucher was created, without the Tropical Voucher’s limitations of a 365- day notification period, and single-transfer ability. In 2014 the differences between the two existing vouchers were eliminated making it possible for both voucher applicants to notify the FDA only 90 days prior to a voucher’s use, and transfer/sell the voucher to other companies unlimited times. Most recently, in 2016, the Countermeasures voucher was signed into law, making it the third voucher available, specifically targeting drugs that prevent or treat harm from a biological, chemical, radiological or nuclear agent.
Conclusion
These expedited programs are open to treatments covering a broad range of diseases; by taking a little extra time to become familiar with them you could save your drug many months of working its way through the pipeline. When thinking about these programs broadly, Fast Track and Breakthrough Therapy are the most similar. The most significant difference between them is the type of data needed: for Fast Track designation you can use preliminary data whereas Breakthrough Therapy designation requires preliminary clinical data. Because of this, Fast Track can be thought of as a junior version of Breakthrough Therapy, especially since the FDA commits greater resources and attention to handling Breakthrough drugs. The Accelerated Approval program differs from the others as it brings the drug to market, rather than just expediting the process – the FDA considers the severity and rarity of the disease and the availability of current treatments. The Priority Review is best thought of as putting your drug at the top of the ‘to be reviewed’ pile.
Surprisingly, while the voucher programs have recently expanded and removed limitations, the FDA’s drug expediting tracks have remained the same over the last several years. The backlogs caused by the COVID-19 pandemic may make change necessary. Until that change comes, the best way to move forwards with your drug, in spite of the pandemic-related effects, is to take advantage of the tracks outlined in this article.
References
- Census Bureau, (2020). Older Americans Month: May 2021. https://www.census.gov/newsroom/stories/older-americans-month.html
- James, Julia (2010). Why drug development is time consuming and expensive (hint: it’s hard). Scope, published by Stanford Medicine. https://scopeblog.stanford.edu/2010/07/21/mochly_rosen_lecture/
- Ogbru, Omudhome (2020). Why Drugs Cost So Much. MedicineNet. https://www.medicinenet.com/drugs_why_drugs_cost_so_much/views.htm
- Peterson, Peter G. (2020). Why are Americans Paying More For Healthcare? https://www.pgpf.org/blog/2020/04/why-are-americans-paying-more-for-healthcare
- Rowe, Sebastian (2020). Modern Drug Discovery: Why is the drug development pipeline full of expensive failures? SITN (Science in the News: a Graduate Student Group at the Harvard Graduate School of the Arts and Sciences). https://sitn.hms.harvard.edu/flash/2020/modern-drug-discovery-why-is-the-drug-development-pipeline-full-of-expensive-failures/#
- Vitale, James (2019). Animal Models in Drug Discovery. Taconic. https://www.taconic.com/taconic-insights/quality/animal-models-drug-discovery.html#footnote
- Wilson-Sanders, S.E. (2011). Invertebrate Models for Biomedical Research, Testing, and Education, ILAR Journal, 52 (2) 126-152, https://doi.org/10.1093/ilar.52.2.126
Embrace the possibilities of drug discovery and development. Get in touch with us today and tap into our comprehensive services to revolutionize the landscape of healthcare.



