Summary:
Organ and tissue regeneration is an area of research with a potential impact on millions of patients who have suffered traumas as diverse as heart attacks, strokes, or amputation. So, how is this field investigated? Look no further than the zebrafish, which has an astonishing capacity for organ and appendage regrowth and could unlock the possibilities of human tissue regeneration.
Tissue damage can occur through injury or disease processes, and is responsible for symptoms and mortality associated with many serious health conditions. For example, when blood flow to the heart is lost during a heart attack, the heart muscle cells (cardiomyocytes) suffer damage and may die. As the heart heals, it develops scar tissue rather than regenerating the cardiomyocytes, causing loss of function (1). Tissue damage is also a characteristic of multiple other diseases including strokes, neurodegenerative disorders, and diabetes. Humans have limited capacity for organ regeneration – only the skin and liver are capable of regaining full form and function. Cells such as cardiomyocytes and neurons have limited or no regeneration in adults, and scar tissue is formed rather than the normal cells of the organ (2).
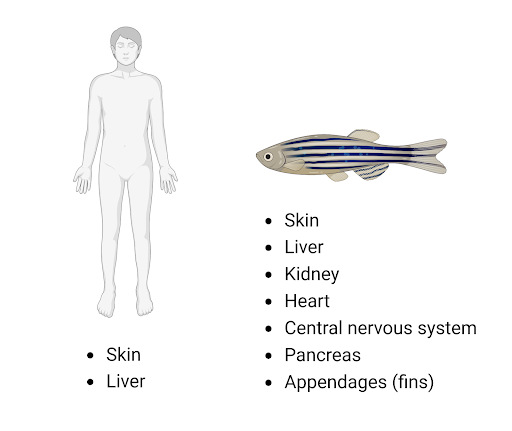
Figure 1. Fully regenerating organs in humans and zebrafish (created with BioRender).
In contrast to humans, zebrafish can fully regenerate most organs, including the heart, brain, spinal cord, and appendages [Figure 1]. Organs such as the caudal (tail) fin can even regenerate multiple times in response to successive amputations. Due to the ease of the amputation process and the limited effects on the fish during the healing process, caudal fin ablation is a popular model to study tissue regeneration in zebrafish. Furthermore, the fin has limited pigment cells, allowing for in vivo visual monitoring of cellular growth during the healing process. The caudal fin reconstructs lost tissue through a process called epimorphic regeneration [Figure 2] (3). First, an epithelial structure (wound epidermis) forms at the amputation site. Next, small masses of progenitor cells (blastemas) proliferate under the wound epidermis. New tissue is formed from differentiated cells in the blastema. A few weeks after amputation, the fin is fully restored of its lost tissue, with complete function and at the same size as the original tissue (4). Although zebrafish may regenerate organ types that humans cannot, the conserved mechanisms underlying zebrafish regeneration give hope that someday these processes can be induced in humans to repair injuries and disease-damaged tissue. For example, the Wnt/ꞵ-catenin pathway is necessary for cell proliferation in the blastema and is conserved in organisms ranging from planaria to mammals (5). The conservation of genes and organ functions between humans and zebrafish make zebrafish an attractive model for basic regeneration research, as well as for the development of novel drugs and therapies for tissue growth.
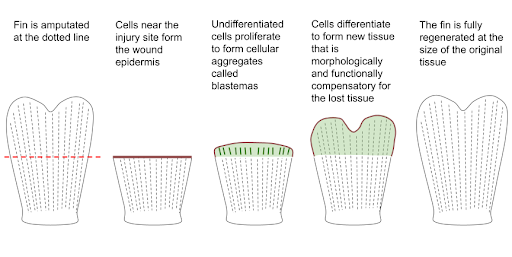
Figure 2. Epimorphic regeneration fully restores amputated fin tissue.
How can you use zebrafish to study regeneration? In healthy fish, tissue can be injured or removed to begin the regeneration process. Surgical removal or cryoinjury (using super-cooled instruments) of tissue are both commonly used to study fin and heart regeneration.2 Other injuries are caused by injection or injection of chemicals such as acetaminophen, which causes liver injury.6 Additionally, specific populations of cells can be genetically targeted for removal by the expression of cytotoxic proteins.2 Pancreatic regeneration is often initiated using ablation via metronidazol or nifurpirinol; these drugs are metabolized into cytotoxins by nitroreductase and cause nitroreductase-expressing pancreatic beta cells to undergo apoptosis.7 The zebrafish further lends itself to tissue regeneration studies by its genetic tractability and the relative ease of generating knockout and transgenic animals. Knockdown or knockout of genes involved in tissue repair may elucidate their regenerative functions, while reporter lines allow researchers to track specific cell types (e.g. immune cells like macrophages or neutrophils) during the wound healing process. The ease of generating zebrafish models, as well as the numerous other benefits of working with zebrafish, make zebrafish an ideal system for studying organ and tissue regeneration.
Click here to learn about InVivo Biosystems’ KO capabilities, or reach out to one of our experts today.
References
- What is a heart attack? American Heart Association https://www.heart.org/en/health-topics/heart-attack/about-heart-attacks
- Marques, I. J., Lupi, E. & Mercader, N. Model systems for regeneration: zebrafish. Development 146, dev167692 (2019).
- Pfefferli, C. & Jaźwińska, A. The art of fin regeneration in zebrafish. Regen. Oxf. Engl. 2, 72–83 (2015).
- Sehring, I. M. & Weidinger, G. Recent advancements in understanding fin regeneration in zebrafish. WIREs Dev. Biol. 9, e367 (2020).
- Wehner, D. et al. Wnt/β-Catenin Signaling Defines Organizing Centers that Orchestrate Growth and Differentiation of the Regenerating Zebrafish Caudal Fin. Cell Rep. 6, 467–481 (2014).
- Cox, A. G. et al. S-nitrosothiol signaling regulates liver development and improves outcome following toxic liver injury. Cell Rep. 6, 56–69 (2014).
- Bergemann, D. et al. Nifurpirinol: a more potent and reliable substrate compared to metronidazole for nitroreductase-mediated cell ablations. Wound Repair Regen. 26, 238–244 (2018).
Take advantage of our advanced zebrafish toxicity testing service today and discover the cutting-edge solution for evaluating the safety and potential risks of chemicals and compounds.