Summary
Nobody thought in the early 20th century, a fish model system would revolutionize and contribute to the scientific community in such a way that zebrafish did as of today. It’s all because of George Stresinger’s pioneering idea to engineer zebrafish into a new and suitable model system that is compatible with human diseases other than phage systems. With their relatively short lifespan, transparent embryonic development, and high gene similarity to humans (70%), Streisinger and his colleagues found the model organism they were looking for. Although the zebrafish is now one of the most popular fish models used in research, there are other fish species (such as the medaka, goldfish, carp, and Antarctic fish) that have their own benefits to research. In this article, we will review the history of the zebrafish, and discuss the scenarios when other fish species are advantageous.
The Beginnings of Zebrafish Research
Zebrafish (Danio rerio) research started in the early 1960s when scientists wanted to branch out from their hefty reliance on the phage systems, which was one of the go-to model systems during those days (yourgenome, 2021). In particular, scientists wanted a more complex model that would enable them to understand brain functions, neuronal networks, and other biomedical research. At this time, Seymour Benzer started his research of the nervous systems of Drosophila melanogaster, and Sydney Brenner began his studies on C. elegans (recipient of Nobel prize in physiology or medicine “for their discoveries concerning genetic regulation of organ development and programmed cell death” jointly with H.Robert Horvitz and Sir John E. Sulston) (Bonini, 2008; Cold Spring Harbor Laboratory, 2019). George Streisinger, however, deviated slightly from his fellow scientists to focus on vertebrate model organisms, which, due to their complexity, had significant advantages over the other model systems.
Prequel to Streisinger’s Studies
In 1822, during the British colonization era of India, Scottish Physician Francis Hamilton described the beautiful, yet small fish that he captured in the River Kosi near Nathpur, India, and named it Cyprinus rerio, (Hamilton, 1822; Zebrafish Rock, 2020a). Later, this fish became known as Danio rerio; Danio derives from the Bengali word “Dhani,” which translates to “of the rice fields” (Zebrafish Rock, 2020c).
Figure 1. Source: https://twitter.com/ZebrafishRock/status/1275509856884133893/photo/1
Danio rerio also has been called by its homotypic synonym Brachydanio rerio, in some initial research articles (Simonetti et al., 2015; Schoch et al., 2020). By 1934, Charles Creaser had the idea to use zebrafish as a model organism in embryology studies and had published an article describing the handling of the zebrafish where he emphasized the ease of research studies of this species (Varga, 2016). The research community was slow to adopt zebrafish as a model organism, however, and over the next few decades other fish model systems, such as the medaka, carp, and goldfish surpassed the number of studies using zebrafish (Kwitek, 2008).
This all changed in the 1960s when Streisinger and his assistant Charline Walker started maintaining and breeding zebrafish in his lab at The University of Oregon (Varga, 2016). To start this colony Streisinger handpicked a bunch of zebrafish from pet shops near the University of Oregon (Creaser, 1934). The zebrafish’s small size, external fertilization, and fast development made it an ideal model species and a prominent choice to study vertebrates among developmental biologists. The aforementioned landmark paper “Production of clones of homozygous diploid zebrafish (Brachydanio rerio)” by Streisinger and colleagues sparked interest in zebrafish, not only from the scientific community but also from the general public as well. For instance, here is a cartoon from the Chicago Tribune (Varga, 2016).
Figure 2. The Chicago Tribune depicts George Streisinger. Source: https://thenode.biologists.com/doctor-delayed-publications-remarkable-life-george-streisinger/careers/#comments
After several highly publicized findings using zebrafish, the zebrafish has become a unique animal model in basic research, as well as a prominent model of choice in Bio-tech research. Specifically, it is now commonly used in biochemical and gene editing studies, and for establishing human disease models (Li et al., 2013).
The Zebrafish Genome Project
Another milestone in zebrafish research was the zebrafish genome project which was completed at the Wellcome Trust Sanger Institute (2001-2013). This project resulted in over 26,000 protein-coding genes being sequenced, at the best quality possible. This is the largest gene set of any vertebrate sequenced so far and has been an invaluable reference tool for scientists (yourgenome, 2016). Interestingly, zebrafish share 70% of the same genes as humans, and around 84% of human genes known to be associated with the disease can be studied in zebrafish. Additionally, zebrafish’s major organs and tissues also function like humans (Gilbert, 2016). Since the zebrafish have been sequenced to the best quality possible, scientists could able to develop nearly 15,000 mutations in the zebrafish (Lo J et al., 2003). Typically, researchers study first the genetic mutations associated with a particular human disorder, then, these mutations are reproduced in zebrafish and observed as they develop. By utilizing zebrafish as subjects of pharmacological studies, and as diagnostic measures, significant progress in understanding and treatment in several diseases have been achieved, such as cancers, and cardiovascular diseases.
The Advantages of a Zebrafish Model System
Researchers can easily visualize embryo development using zebrafish as a model system because zebrafish are transparent from the one-cell stage until the 5 dpf (days post-fertilization), and external development. Because of its transparency, all the developments occurring during this time would be easy to follow and the scientists are heavily relying on different stages, such as the CRISPR/cas9 and Tol2 injections would be ideal to conduct at a one-cell stage. This allows scientists to follow the development from the first moments of fertilization through to free swimming, actively feeding larval fish. Baby zebrafish hatch 2 dpf and begin actively feeding 5 dpf. During the first 5 dpf, all major organ systems develop and begin functioning. Furthermore, zebrafish can breed year-round with females capable of producing several hundred embryos each week. The abundance of offspring is especially advantageous for genetic mapping studies and experiments that demand large sample sizes (Veldman & Lin, 2008).
Learn more about our zebrafish genome editing services
Alternative fish models to study targeted human diseases
Medaka (Japanese ricefish)
Commonly known as medaka, the Oryzias latipes is a small, freshwater, fish that is egg-laying and bony, and found primarily in Asian countries, such as Japan, Korea, and China (Shima & Mitani, 2004). It is a eurythermal fish, with a survival threshold higher than other fish species – medaka can survive in an outdoor pond with a temperature ranging from 0oC to 400C. The idea to use medaka in research first came about in 1975, when T. Yamamoto, of Nagoya University, and his colleagues published a book titled ‘Medaka (Killifish)-Biology and Strains’ (Shima & Mitani, 2004). This book paved the way for other scientists to explore the potentiality of Medaka as one of the potential model organisms (Shima & Mitani, 2004).

Figure 3: Medaka fish. Source: https://www.noldus.com/blog/japanese-medaka-toxicology
One of the important findings from medaka research studies was that this fish’s genome contains the element Tol2 (active DNA-based transposable element), and has DNA sequences similar to those of transposons of the hAT (hobo/Activator/Tam3) family (Kawakami & Shima, 1999), which is a superfamily comprising of all the transposable elements among the wide range of organisms. Tol2 would be expected to be active if introduced into mammals. The first transposon was reported by Barbara McClintock, which got her a Nobel prize in 1983 for her discovery of mobile genetic elements. In medaka fish, Tol2 transposable element was cloned from the tyrosinase gene locus of the i4 allele of the albino mutant fish. By optimizing the Tol2 transposons, we can pioneer this technology in other model organisms, such as in zebrafish genome editing, and excision in human and mouse cells in culture (Koga et al., 2003). Furthermore, medaka fish have been used in studying bone diseases, fluorescent tagging, large-scale mutagenesis screens, and several disease phenotypes (Schartl, 2014). Like zebrafish, medaka fish can be studied using both CRISPR/Cas9 and Tol2 transgenesis. Medaka is considered a preferred alternative to Zebrafish research depending on its usage applications.
Learn more about Zebrafish Tol2 transgenesis
African turquoise killifish (Nothobranchius furzeri)
In 1969, the African turquoise killifish which was a native to East Africa initially got its species recognition in Zimbabwe and Mozambique (Furzer, 1969; Jubb, 1971; Parle, 1970). However, its research potential remains unnoticed for decades among researchers. Later in 2003, Alessandro Cellerino’s research group at the Scuola Normale Superiore, Italy, identified its research potential especially in aging studies because of its short life span which was extended from 2 months of original life span to 4 months because of the constant improvement in the killifish husbandry across different research groups (Polacik et al., 2016; Reichwald et al., 2015; Valenzano et al., 2015).
Killifish does have a unique feature that sets it apart from other fish model organisms by possessing a short lifespan which is critical for its adaptability and survivability during the rainy season in its natural habitat, and on the other hand, embryos can enter the diapause phase for months during the dry seasons which is much longer than their total lifespan waiting for the rainy season to hatch (Hu, C. K., & Brunet, A. (2018).
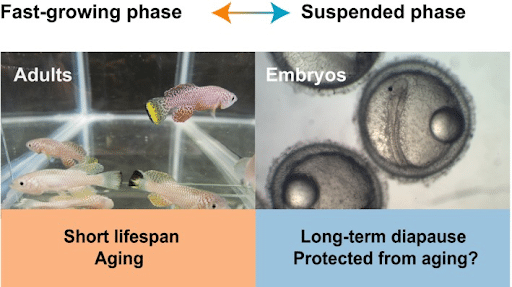
Figure 4. Two different phases of lifespan in killifish (Hu, C. K., & Brunet, A. (2018)
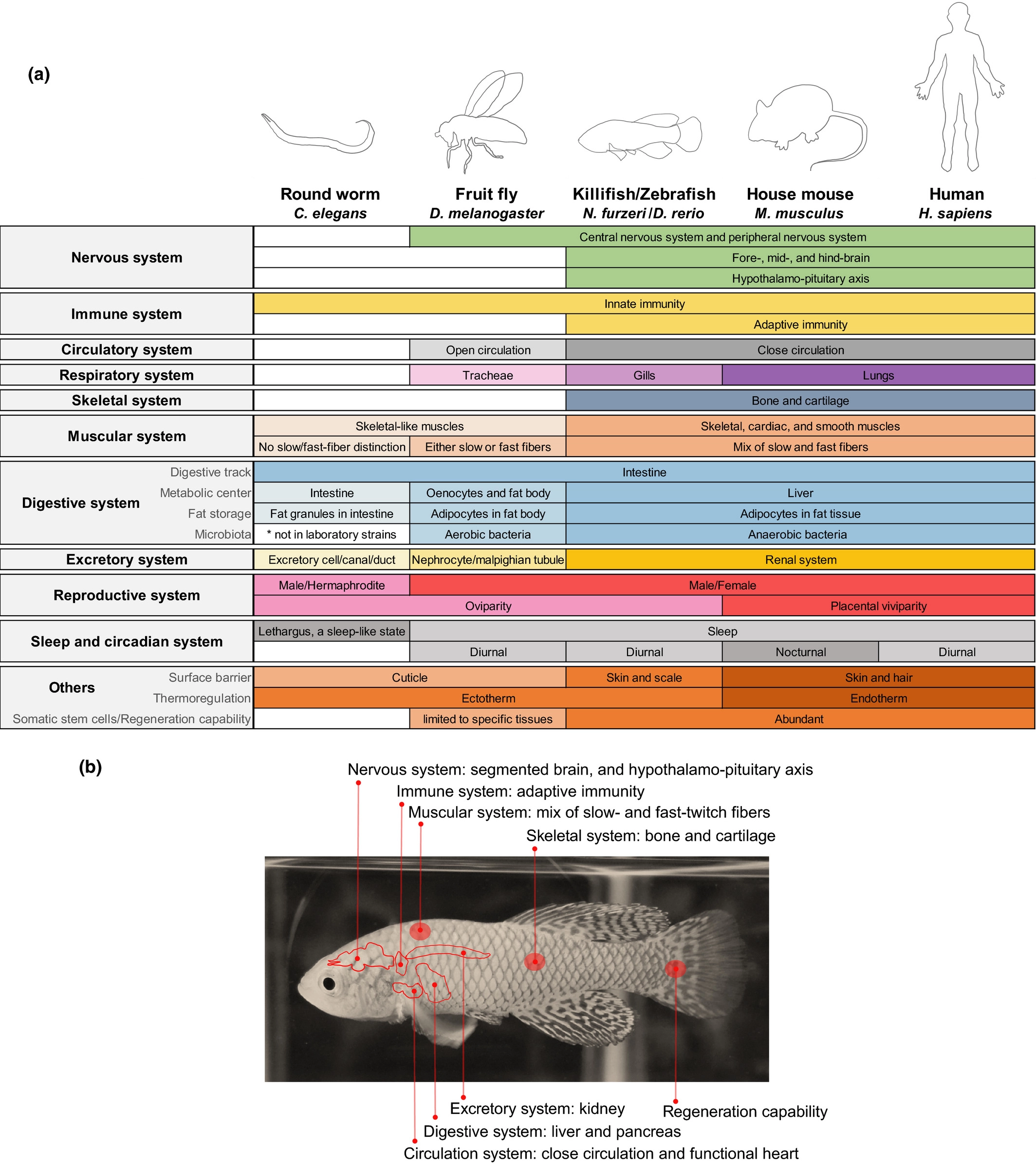
Figure 5. a) Comparison of different human biological features across different model species b) Features of Killifish that are conserved across vertebrate model organism which are utlized in the several human research studies (Hu, C. K., & Brunet, A. (2018)
Antarctic fish
Antarctic fish have also been investigated by researchers to study human diseases, such as osteoporosis and anemia. Antarctic rock cod (Notothenia coriiceps), a subtype of notothenioid fish, possesses a mineralized skeleton, which makes it a prominent candidate in studying the pathology of bone diseases osteoporosis (Schartl, 2014). Another subtype, white-blooded icefish (Channichthyidae) is a suitable model to study human anemias such as thalassemias, sickle cell anemia, and Fanconi anemia; this is because most anemic conditions are characterized by a reduction in erythrocytes number and hemoglobin count in the blood which leads to a lack of oxygen supply in the blood, and icefish usually have no erythrocytes and do not use hemoglobin for oxygen transport. As a result, icefish is a unique candidate to study the Red blood corpuscles formation (Detrich & Yergeau, 2004).
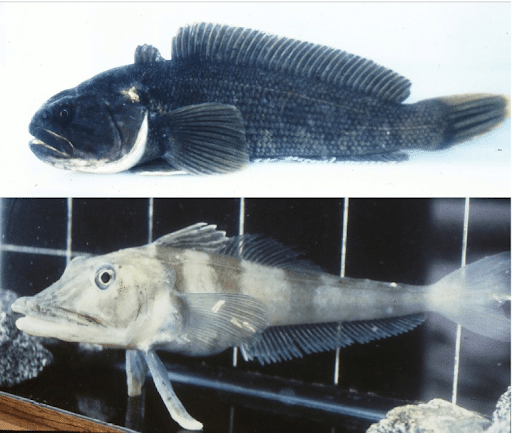
Figure 6: Antarctic Fish. Source: https://journals.biologists.com/dmm/article/7/2/181/4005/Beyond-the-zebrafish-diverse-fish-species-for
Goldfish and carp
Other Lesser-known animal model systems such as goldfish (Carassius auratus) and common carp (Cyprinus carpio) are the most commonly used domesticated aquatic species. Specifically, goldfish have been a promising model for biomedical research with mutant strains exhibiting morphologies of eyes, fins, skeleton, body shape, coloration, etc.- some of which resemble human diseases (Omori & Kon, 2019). Research studies show that the goldfish and carp are both derived from a common ancestor via a lineage-specific genome duplication event (Kuang, YY et al, 2016). Hybrid goldfish (Goldfish * carp) would provide an ideal candidate that will be resistant to cyprinid herpesvirus-2 (CyHV-2) which could help to develop a viral resistant fish strain and less concern in lethality issues in the domestication market (Bergmann, et al., 2010).
Conclusion
The zebrafish has had a fascinating journey from the fish tanks of pet shops to becoming one of the most useful models in biomedical research. Largely pioneered by George Streisinger and his colleagues, the zebrafish has gone on to worldwide recognition as a complex, cheaper, and more space-saving model than its mammalian counterparts. Specifically, zebrafish are valuable models of human disorders, thanks to their ability to be easily genetically modified. This being said, we cannot ignore the importance of other fish species in research studies, such as the Medaka, Antarctic fish, Goldish, Koi, carp, Cichlid (in the studies of craniofacial developmental disorders), Blind cavefish (retinal degeneration, albinism, and sleep disorders), Annual killifish (models for aging), and Toadfish (hepatic encephalopathy and sickle cell anemia). Each fish species has its unique advantages over other fish. Excitingly, fish model research studies are just in their infancy, as we are still a long way from using these different fishes to their full potential.
References
- Baumgart, M. , Groth, M. , Priebe, S. , Savino, A. , Testa, G. , Dix, A. , … Cellerino, A. (2014). RNA‐seq of the aging brain in the short‐lived fish N. furzeri ‐ conserved pathways and novel genes associated with neurogenesis. Aging Cell, 13, 965-974. 10.1111/acel.12257
- Bergmann, S. M., Sadowski, J., Kiełpiński, M., Bartłomiejczyk, M., Fichtner, D., Riebe, R., Lenk, M., & Kempter, J. (2010). Susceptibility of koi x crucian carp and koi x goldfish hybrids to koi herpesvirus (KHV) and the development of KHV disease (KHVD). Journal of fish diseases, 33(3), 267-272. https://doi.org/10.1111/j.1365-2761.2009.01127.x
- Bonini N. M. (2008). A tribute to Seymour Benzer, 1921–2007. Genetics, 180(3), 1265-1273. https://doi.org/10.1534/genetics.104.97782
- Cold Spring Harbor Laboratory (2019). Sydney Brenner – In Memorial. Library Cold Spring Harbor Laboratory. http://library.cshl.edu/pages/Sydney-Brenner-Memorial/
- Creaser, C.W. (1934) The technic of handling the zebra fish (Brachydanio rerio) for the production of eggs which are favorable for embryological research and are available at any specified time throughout the year. Copeia. 4:159-161.
- Detrich, H. W., 3rd, & Yergeau, D. A. (2004). Comparative genomics in erythropoietic gene discovery: synergisms between the Antarctic icefishes and the zebrafish. Methods in cell biology, 77, 475-503. https://doi.org/10.1016/s0091-679x(04)77026-0
- Furzer, R. E. (1969). A Rhodesian hobbyists report. Tropical Fish Hobbyist, 17, 24-27
- Gilbert, Scott (2016). Zebrafish help researchers study human genes. Penn State News. https://news.psu.edu/story/418819/2016/07/28/research/zebrafish-help-researchers-study-human-genes
- Hamilton, Francis. (1822). An account of the fishes found in the river Ganges and its branches. Vol. 1. Archibald Constable.
- Hoppe, B. , Pietsch, S. , Franke, M. , Engel, S. , Groth, M. , Platzer, M. , & Englert, C. (2015). MiR‐21 is required for efficient kidney regeneration in fish. BMC Developmental Biology, 15, 43 10.1186/s12861-015-0089-2
- Hu, Chi-Kuo, and Anne Brunet. “The African turquoise killifish: A research organism to study vertebrate aging and diapause.” Aging cell vol. 17,3 (2018): e12757. doi:10.1111/acel.12757
- Jubb, R. A. (1971). A new Nothobranchius (Pisces, Cyprinodontidae) from Southeastern Rhodesia. Journal of the American Killifish Association, 8, 12-19
- Kawakami, K., & Shima, A. (1999). Identification of the Tol2 transposase of the medaka fish Oryzias latipes that catalyzes excision of a nonautonomous Tol2 element in zebrafish Danio rerio. Gene, 240(1), 239-244. https://doi.org/10.1016/s0378-1119(99)00444-8
- Keane, M. , Semeiks, J. , Webb, A. E. , Li, Y. I. , Quesada, V. , Craig, T. , … de Magalhaes, J. P. (2015). Insights into the evolution of longevity from the bowhead whale genome. Cell Reports, 10, 112-122. 10.1016/j.celrep.2014.12.008
- Koga, A., Iida, A., Kamiya, M. et al. (2003). The medaka fish Tol2 transposable element can undergo excision in human and mouse cells. J Hum Genet 48, 231-235. https://doi.org/10.1007/s10038-003-0016-4
- Kwitek A. E. (2008). A guinea pig’s history of biology. The Journal of Clinical Investigation, 118(5), 1589. https://doi.org/10.1172/JCI35743
- Kuang, YY., Zheng, XH., Li, CY. et al. The genetic map of goldfish (Carassius auratus) provided insights to the divergent genome evolutions in the Cyprinidae family. Sci Rep 6, 34849 (2016). https://doi.org/10.1038/srep34849
- Li, H. H., Huang, P., Dong, W., Zhu, Z. Y., & Liu, D. (2013). Yi chuan = Hereditas, 35(4), 410-420. https://doi.org/10.3724/sp.j.1005.2013.00410
- Lo J, Lee S, Xu M, et al. 15000 unique zebrafish EST clusters and their future use in microarray for profiling gene expression patterns during embryogenesis. Genome Res. 2003;13(3):455-466. doi:10.1101/gr.885403
- Omori, Y., & Kon, T. (2019). Goldfish: an old and new model system to study vertebrate development, evolution and human disease. Journal of biochemistry, 165(3), 209-218. https://doi.org/10.1093/jb/mvy076
- Parle, R. (1970). Two new Nothos from Rhodesia. AKA Killi Notes, 3, 15-21.
- Polacik, M. , Blazek, R. , & Reichard, M. (2016). Laboratory breeding of the short‐lived annual killifish Nothobranchius furzeri . Nature Protocols, 11, 1396-1413. 10.1038/nprot.2016.080
- Reichwald, K. , Petzold, A. , Koch, P. , Downie, B. R. , Hartmann, N. , Pietsch, S. , … Platzer, M. (2015). Insights into sex chromosome evolution and aging from the genome of a short‐lived fish. Cell, 163, 1527-1538. 10.1016/j.cell.2015.10.071
- Schartl M. (2014). Beyond the zebrafish: diverse fish species for modeling human disease. Disease models & mechanisms, 7(2), 181-192. https://doi.org/10.1242/dmm.012245
- Schoch, C. L., Ciufo, S., Domrachev, M., Hotton, C. L., Kannan, S., Khovanskaya, R., Leipe, D., Mcveigh, R., O’Neill, K., Robbertse, B., Sharma, S., Soussov, V., Sullivan, J. P., Sun, L., Turner, S., & Karsch-Mizrachi, I. (2020). NCBI Taxonomy: a comprehensive update on curation, resources and tools. Database : the journal of biological databases and curation, 2020, baaa062. https://doi.org/10.1093/database/baaa062
- Shima, A., & Mitani, H. (2004). Medaka as a research organism: past, present and future. Mechanisms of development, 121(7-8), 599-604. https://doi.org/10.1016/j.mod.2004.03.011
- Simonetti, R.B., Marques, L.S., Streit Jr, D.P., Oberst, E.R. (2015). ZEBRAFISH (Danio rerio): THE FUTURE OF ANIMAL MODEL IN BIOMEDICAL RESEARCH. Journal of FisheriesSciences.com https://www.fisheriessciences.com/fisheries-aqua/zebrafish-danio-rerio-the-future-of-animal-model-inbiomedical-research.php?aid=6681
- Valenzano, D. R. , Benayoun, B. A. , Singh, P. P. , Zhang, E. , Etter, P. D. , Hu, C. K. , … Brunet, A. (2015). The African turquoise killifish genome provides insights into evolution and genetic architecture of lifespan. Cell, 163, 1539-1554. 10.1016/j.cell.2015.11.008
- Valenzano, D. R. , Terzibasi, E. , Cattaneo, A. , Domenici, L. , & Cellerino, A. (2006a). Temperature affects longevity and age‐related locomotor and cognitive decay in the short‐lived fish Nothobranchius furzeri . Aging Cell, 5, 275-278. 10.1111/j.1474-9726.2006.00212.x
- Valenzano, D. R. , Terzibasi, E. , Genade, T. , Cattaneo, A. , Domenici, L. , & Cellerino, A. (2006b). Resveratrol prolongs lifespan and retards the onset of age‐related markers in a short‐lived vertebrate. Current Biology, 16, 296-300. 10.1016/j.cub.2005.12.038
- Varga, Máté (2016). The Doctor of Delayed Publications – the remarkable life of George Streisinger. The Node. https://thenode.biologists.com/doctor-delayed-publications-remarkable-life-george-streisinger/careers/#comments
- Veldman, M. B., & Lin, S. (2008). Zebrafish as a developmental model organism for pediatric research. Pediatric research, 64(5), 470-476. https://doi.org/10.1203/PDR.0b013e318186e609
- Walker, C. (2016). Streisinger Lab. ZFIN. https://zfin.org/ZDB-LAB-980209-8
- Wendler, S. , Hartmann, N. , Hoppe, B. , & Englert, C. (2015). Age‐dependent decline in fin regenerative capacity in the short‐lived fish Nothobranchius furzeri . Aging Cell, 14, 857-866. 10.1111/acel.12367
- Yourgenome (2021). Why use zebrafish in research? Yourgenome’s website, by Wellcome Genome Campus. https://www.yourgenome.org/facts/why-use-the-zebrafish-in-research
- Yourgenome (2016). Tiny fish, big splash: the story of the zebrafish. Yourgenome’s website, by Wellcome Genome Campus. https://www.yourgenome.org/stories/tiny-fish-big-splash-the-story-of-the-zebrafish
- Zebrafish Genome Compared to Human: How Are They Similar. https://blog.biobide.com/zebrafish-as-a-model-organism-in-research-methods-and-benefits
- Zebrafish Rock! [@ZebrafishRock]. (2020, Jun 23). Danio derives from the Bengali word “Dhani,” which translates to “of the rice fields.” [Tweet]. Twitter. https://twitter.com/ZebrafishRock/status/1275509856884133893
- Zebrafish Rock! [@ZebrafishRock]. (2020, Jun 22). Here is the cover of the 1981 issue of @nature containing the landmark paper by Streisinger. [Tweet]. Twitter. https://twitter.com/ZebrafishRock/status/1275146273230860290
- Zebrafish Rock! [@ZebrafishRock]. (2020, Jun 17). Scottish Physician Francis Hamilton first described the “beautiful” but “insipid” #zebrafish [Tweet]. Twitter. https://twitter.com/zebrafishrock/status/1273236730871758849
Harness the power of zebrafish toxicity testing for reliable and efficient evaluation of chemical safety. Contact us today to unlock the potential of this cutting-edge approach and make informed decisions that prioritize safety in your research and development endeavors.


