Summary:
Parkinson’s disease [PD] is a progressive, debilitating condition which affects over 10 million people worldwide. Being such an impactful, and costly disease (~$52 billion per year in the USA), researchers have turned to modeling the disease in alternative animal models like the zebrafish (Parkinson’s Foundation, 2018). In this article we discuss how larval zebrafish are being used to model PD, and why it is such an advantageous model.
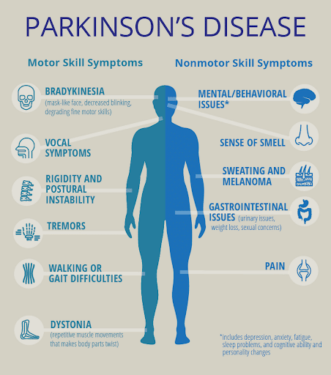
Figure 1. Characterization of Parkinson’s Disease (Singapore Brain-Spine-Nerves Centre, n.a.).
Parkinson’s disease (PD) is a progressive neurodegenerative disease characterized by the impairment and death of dopamine-producing neurons in the substantia nigra. Symptoms include tremors, speech changes, slowed movement, and balance problems. Currently, over a million people in the USA are suffering from PD, and this number is only expected to rise as the population ages. Associated with aging, 96% of affected adults are diagnosed after 50, and complications of the disease can significantly decrease quality of life; patients often experience sleep problems, depression and anxiety, and cognitive difficulties (Parkinson’s Foundation, 2018). Thus, there is a pressing need for better PD-targeted therapeutics. In order to develop said therapeutics, researchers are turning to a larval zebrafish model to gain more insights into this disease.
Larval zebrafish can be a particularly beneficial model of PD phenotypes because the developing zebrafish nervous system has been extensively studied, and, perhaps surprisingly, is homologous to many aspects of the human nervous system. Specifically useful for PD research are the similarities within the human and zebrafish dopaminergic system. The zebrafish ventral telencephalon is considered homologous to the mammalian striatum (Stewart et al., 2014), a brain region that plays a critical role in motor and cognitive behaviors, and is connected to the substantia nigra via dopaminergic projections (Redgrave et al., 2010).
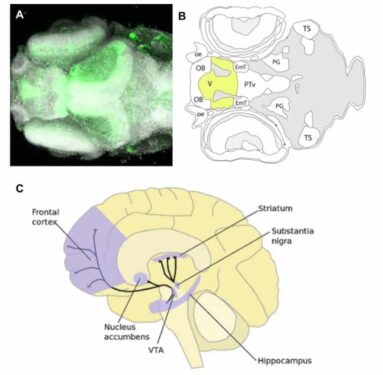
Image 1. (A) Top-down image of a zebrafish ventral telencephalon. (B) Top-down diagram of the zebrafish brain, with ventral telencephalon highlighted. (C) Lateral view of the human brain with striatum and substantia nigra shown (Zebrafish UCL, 2017; Perry, 2015).
Some signs of PD, such as anxiety-related behaviors or changes in movement, can be measured in zebrafish by analyzing swimming behavior and locomotion. Furthermore, zebrafish larvae are transparent, enabling easy imaging of the nervous system, and their large brood size allows researchers to quickly collect large numbers of fish for ‘omics analyses. The availability of automated methods for collecting and analyzing movement data for large sample sizes is an additional benefit of using zebrafish to model PD.
The most straightforward method of modeling PD in fish is to expose them to a neurotoxin; however, exposure must be carefully timed to ensure that the zebrafish have sufficiently-developed nervous systems to recapitulate the phenotypes of PD observed in humans. In particular, locomotion effects from chemical induction of PD have been inconsistently observed across studies, perhaps due to different timing of treatments. Chemical exposure routes must also be considered; the blood-brain barrier forms in zebrafish at 3 days post-fertilization (Barnhill et al., 2020), and the protective chorion covering the unhatched embryo can also inhibit some toxins (Nishimura et al., 2016).
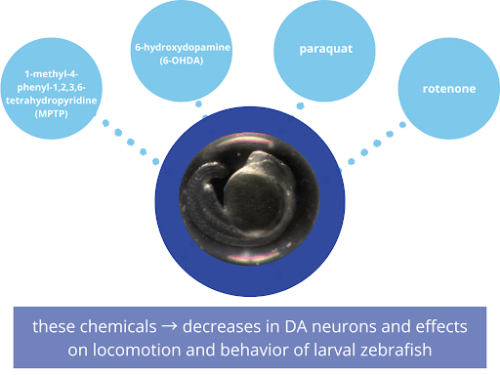
Figure 2. Four commonly-used chemicals for creating a zebrafish model of Parkinson’s disease.
Four common chemicals used to model PD in zebrafish are 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), 6-hydroxydopamine (6-OHDA), paraquat, and rotenone [Figure 2]. These chemicals have caused decreases in DA neurons and effects on locomotion and behavior of larval zebrafish. MPTP is perhaps the most commonly-used chemical; exposure led to decreased DA neurons in the ventral diencephalon and decreased swimming speed in larval zebrafish (Kalyn et al., 2020; Wasel and Freeman, 2020). 6-OHDA has also been used in zebrafish studies, but with slightly varying results. For instance, where Feng et al., observed decreased locomotion and increased time at the bottom of the tank (an anxiety behavior in fish), as well as decreased expression in the DA pathway in the brain, Kalyn et al. observed similar decreased DA expression, but no changes in locomotion (Feng et al., 2014; Kalyn et al., 2020).
As for rotenone and paraquat in zebrafish PD models, Kalyn et al. found that both chemicals decreased DA neurons in the ventral diencephalon but only rotenone caused a change in locomotion (2020). Rotenone can also cause temperature-associated neurotoxicity in zebrafish (Keow, 2016), so any studies using this chemical should be carried out at room temperature rather than the typical zebrafish husbandry temperature of 28.5 °C.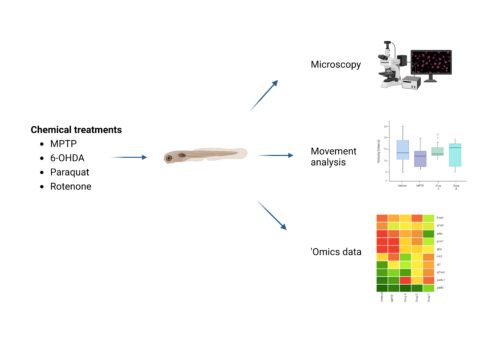 Figure 3. Analyzing PD phenotypes in zebrafish (figure created with BioRender).
Figure 3. Analyzing PD phenotypes in zebrafish (figure created with BioRender).
Modeling PD via neurotoxin exposure is not without limitations, however. While the above-described neurotoxins can induce some signs of PD in zebrafish, the mechanisms by which they do so may be different than those present in PD. To investigate the molecular mechanisms of PD, researchers have used genetic models of PD in zebrafish. PD-related genes such as dj-1, pink1, and prkn can be knocked down by morpholino injection, or knock-out lines created using techniques such as CRISPR (Wasel and Freeman, 2020). Additionally, neurotoxin exposure only produces acute PD signs, not reflecting the chronic and progressive nature of PD (Razali et al., 2021). However, genetic models can be difficult, time-consuming, and expensive to produce, so chemical models of PD in zebrafish still have excellent value as a tool for drug discovery and drug repurposing for PD treatment.
While zebrafish have been studied as a model organism since the 1960s, they are still an underutilized alternative animal model. Their notable benefits, including their transparency, quick development time, large brood size, and extensive anatomical similarities with humans, are useful for many disease models, including PD.
To learn more about zebrafish read:
- Zebrafish 101: A White Paper
- Lost and Found: Zebrafish as a Drug Discovery Tool
- Review of Zebrafish Models to Boost Research in Rare Genetic Diseases
To contact one of our zebrafish experts, click here
References
- Barnhill, L.M., Murata, H., Bronstein, J.M., 2020. Studying the pathophysiology of Parkinson’s disease using zebrafish. Biomedicines 8, 197. https://doi.org/10.3390/biomedicines8070197
- Feng, C.-W., Wen, Z.-H., Huang, S.-Y., Hung, H.-C., Chen, C.-H., Yang, S.-N., Chen, N.-F., Wang, H.-M., Hsiao, C.-D., Chen, W.-F., 2014. Effects of 6-hydroxydopamine exposure on motor activity and biochemical expression in zebrafish (Danio rerio) larvae. Zebrafish 11, 227–239. https://doi.org/10.1089/zeb.2013.0950
- Kalyn, M., Hua, K., Mohd Noor, S., Wong, C.E.D., Ekker, M., 2020. Comprehensive analysis of neurotoxin-induced ablation of dopaminergic neurons in zebrafish larvae. Biomedicines 8, 1. https://doi.org/10.3390/biomedicines8010001
- Keow, J., 2016. Physiological and behavioral changes in a rotenone model of dopamine neurotoxicity and neurodegeneration in zebrafish (Thesis). Université d’Ottawa / University of Ottawa. https://doi.org/10.20381/ruor-339
- Nishimura, Y., Inoue, A., Sasagawa, S., Koiwa, J., Kawaguchi, K., Kawase, R., Maruyama, T., Kim, S., Tanaka, T., 2016. Using zebrafish in systems toxicology for developmental toxicity testing. Congenit. Anom. 56, 18–27. https://doi.org/10.1111/cga.12142
- Razali, K., Othman, N., Mohd Nasir, M.H., Doolaanea, A.A., Kumar, J., Ibrahim, W.N., Mohamed Ibrahim, N., Mohamed, W.M.Y., 2021. The promise of the zebrafish model for Parkinson’s disease: today’s science and tomorrow’s treatment. Front. Genet. 12, 553. https://doi.org/10.3389/fgene.2021.655550
- Redgrave, P., Rodriguez, M., Smith, Y., Rodriguez-Oroz, M.C., Lehericy, S., Bergman, H., Agid, Y., DeLong, M.R., Obeso, J.A., 2010. Goal-directed and habitual control in the basal ganglia: implications for Parkinson’s disease. Nat. Rev. Neurosci. 11, 760–772. https://doi.org/10.1038/nrn2915
- Stewart, A.M., Braubach, O., Spitsbergen, J., Gerlai, R., Kalueff, A.V., 2014. Zebrafish models for translational neuroscience research: from tank to bedside. Trends Neurosci. 37, 264–278. https://doi.org/10.1016/j.tins.2014.02.011
- Wasel, O., Freeman, J.L., 2020. Chemical and genetic zebrafish models to define mechanisms of and treatments for dopaminergic neurodegeneration. Int. J. Mol. Sci. 21, 5981. https://doi.org/10.3390/ijms21175981
- Singapore Brain, Spine, Nerves Center (n.a.). PARKINSON’S DISEASE. https://www.drprempillay.org/brain/parkinsons-disease/
- Parkinson’s Foundation (2018). Statistics. https://www.parkinson.org/understanding-parkinsons/statistics
- Perry, S. (2015). Dopamine and Movement, Brain Facts, https://www.brainfacts.org/Thinking-Sensing-and-Behaving/Movement/2015/Dopamine-and-Movement
- Zebrafish UCL (2017). SUBPALLIUM/ VENTRAL TELENCEPHALON. Zebrafish UCL, http://zebrafishucl.org/forebrain-regions/ventral-telencephalon
Take advantage of our cutting-edge drug toxicity testing in zebrafish today and revolutionize your research and development process.




