Summary
In the scientific community there is an emphasis on positive results: getting published, having a drug be approved, etc. However, crucial learning happens when experiments don’t work – in fact, these “failures” may be some of the most important learning experiences. In this article we discuss the need to shift the focus from failing less, to failing faster and some of the resources available to researchers so they can expedite their own work.
I have not failed. I’ve just found 10,000 ways that won’t work.
– Albert Einstein
Often, when people are introduced to the discipline of science they mistakenly think that in order to be a good scientist their experiments should always show significant results. Scientific progress, however, is made through numerous trials and errors that allow researchers to learn, and ultimately accommodate for, when designing their next experiment and ultimately, making their next discovery.
Thus, what can help the advancement of science is finding ways to speed up the process of discovering what doesn’t work. There are several ways to do this, for instance: using alternate model organisms that have shorter lifespans and are faster to test than traditional rodent models, and/or collaborating with biotech companies that can provide these models or an accelerator lab space.
A multi-model approach to research is an excellent way to shorten the timespan of a study, as some models have a vastly different lifecycle than others (Figure 1). This shorter timespan allows researchers to determine whether their experiment is producing meaningful results, or if their time would be better spent on something else. In this way, non-mammalian models are extremely useful as a proof of hypothesis before deciding to conduct the experiment on more expensive, time-consuming models. For example, if you were to test a hypothesis in C. elegans, and it failed, then there would be no reason to run the experiment in mice. You would have shaved months off of your timeline, allowing you to fail faster. Depending on the research, these non-mammalian models can most suitably be used as a first step before testing on mice, or, they can replace rodent models altogether.
Learn more about InVivo Biosystems’ Models 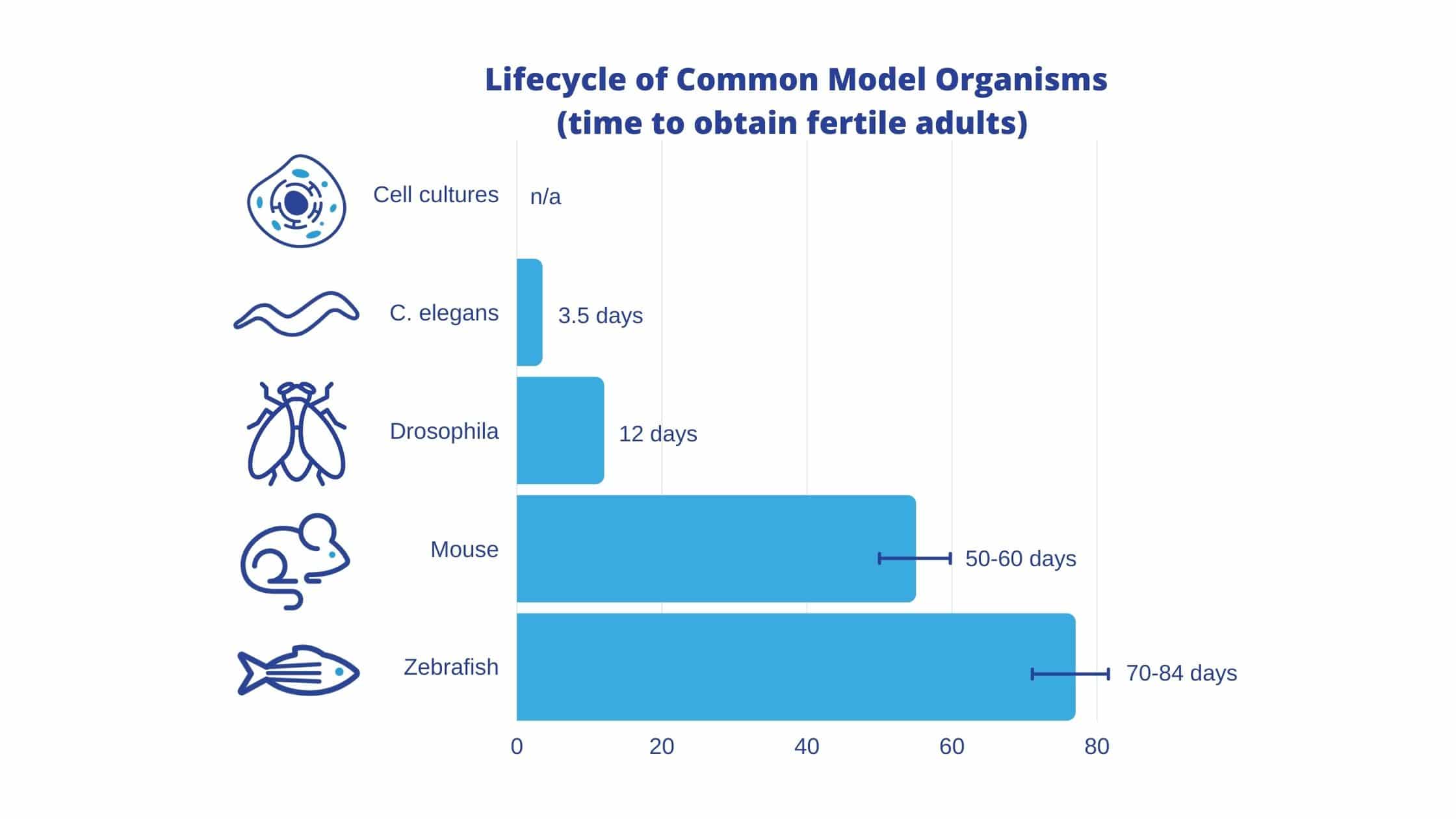
Figure 1. Life Cycle of Common Model Organisms (time to obtain fertile adults)
As Dr. Daniel Starr, UC Davis, said in a recent 17 Minutes of Science interview, collaboration is key, “because [InVivo Biosystems] can crank the transgenics out faster than we can. And now we can use our skills to analyze it in our assays of nuclear movements and things like that.” Working closely together, companies, such as InVivo Biosystems, can help researchers move products forward by providing the models needed so that researchers can focus on what they do best.
There are many different ways to collaborate though. For instance, some companies specialize in greasing the wheels of the manufacturing and approval process. Dr. Christine Kressirer, senior director of Azzur Cleanrooms on Demand, explained that, “cleanrooms allow [researchers] to fail faster” by providing the space and all the supporting services needed to bring a drug to market.
To create a new drug from start to finish, most of them fail and they can fail for many reasons. And it’s not always the science.
Over the course of the past year we have all experienced how impactful the speed of research can be: the COVID-19 vaccine was able to be in phase 3 clinical trials in six months instead of the typical two years. This feat was thanks to previous work on other coronaviruses, mass production while trials were still underway, and the effort to have it bypass red tape which could otherwise have held it up for years (Northwell Health, 2020). The ability to design the three levels of trials at the onset of testing, rather than having to wait for approval before designing the next phase, meant that if the vaccine had failed the second or third phase, researchers wouldn’t have wasted valuable time.
Normally, phase trials run sequentially, to speed up the process, the F.D.A. allowed trials to run simultaneously.
– Bill Whitaker (60 Minutes, 2020)
The next few decades are poised to bring vast advancements in personalized medicine and in vaccine development, as well as many other areas of research. These advancements, however, will not just be thanks to developments in technology, but will also be due to innovations in the infrastructure used for this research. So, in researching let’s be open to collaboration, to finding new ways of running studies: let’s work on failing faster.
References
- Northwell Health (2020). Coronavirus Digital Resource Center. https://www.northwell.edu/coronavirus-covid-19/vaccine/frequently-asked-questions
- Whitaker, Bill (2020) How the Pfizer-BioNTech COVID-19 vaccine was developed. CBS, 60 Minutes.https://www.cbsnews.com/video/covid-vaccine-pfizer-biontech-collaboration-60-minutes-2020-12-20/



