What is the microbiome? How does it help or hinder health? And, what does recent research indicate?
We’ve all had that regret: “oh, I should not have eaten that.” But recently, research has shown that your discomfort can be an indicator of your gut microbiome’s homeostasis. The human
microbiome refers to the combination of microbes and their genes (bacteria, fungi, protozoa and viruses) that exist inside and outside of the human body [1]. Consisting of 10-100 trillion cells, the human microbiome which is essential for digestion has many health benefits ranging from regulating our immune system to producing the vitamins which are needed for blood coagulation. While microbes colonize most surfaces on our bodies: skin, nasopharynx, oral cavity, urogenital tract, and
gastrointestinal tract, the vast majority of humans’ interaction with our microbes occurs in the gastrointestinal gut.
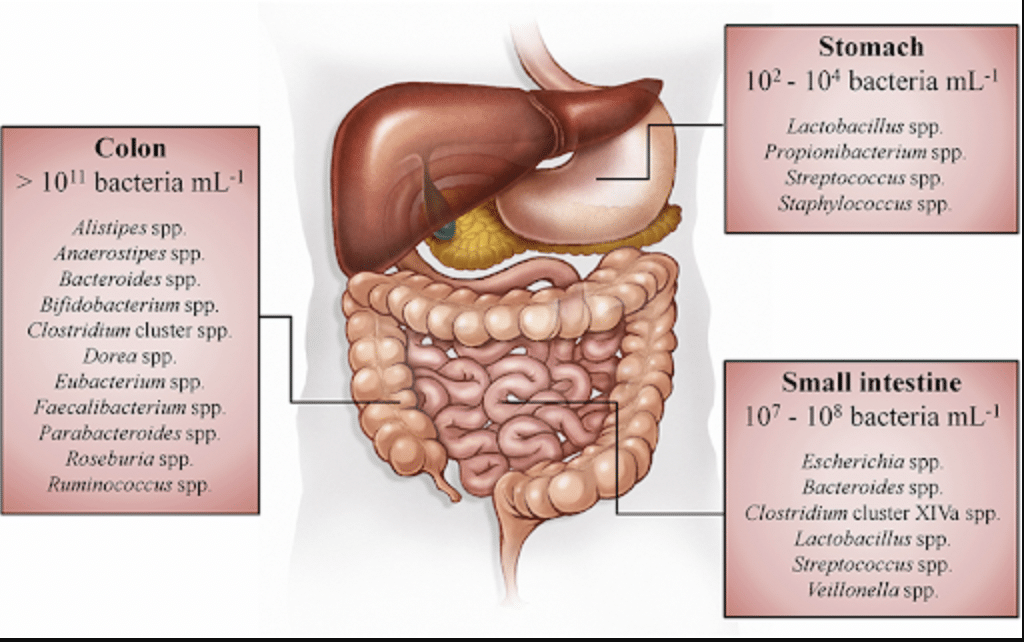
The human intestine harbors at least 100x more bacteria than the entirety of our embryo-derived cells. As a result, these bacteria (or to be more exact “microbiome”) can have a profound impact on our health. This number of microbes is startling, however, what determines a person’s place on the disease-to-health spectrum is not the number of bacteria, but how diverse these bacteria are. The types of bacteria found in our gut are stratified between the intestine and colon [1]. In the intestine, the microbes that reside there are optimized to digest easy to metabolize compounds (simple sugars, fats, amino acids, and other molecules); while in the colon, the resident microbes specialize in fermenting digestio
n of complex carbohydrates [2
].
Figure 1. Spatial distribut
ion and concentrations of bacteria along the gastrointestinal tract of humans [3]
It’s true that the microbiome includes both symbiotic and pathogenic microbes – in a healthy person, these ‘bugs’ coexist without causing harm. However, when there is an abnormality in this microbial ecosystem, known as dysbiosis, a person can be at higher risk of developing diseases such as nonalcoholic fatty liver disease, cardiovascular disease, obesity, diabetes, depression, Parkinson’s disease, autism, and various gastrointestinal cancers [4]. Because of this, there has been a recent increased interest in how microbe populations influence each other (microbe-microbe intera
ctions). And also how microbes, especially pathogenic microbes, sustain themselves inside people (host-microbe interactions).
Figure 2. Social interactio
ns among microbes. Microbes frequently interact with clonemates or other microbes of the same or different species (represented by microbes of different colors and shapes, respectively) [5].
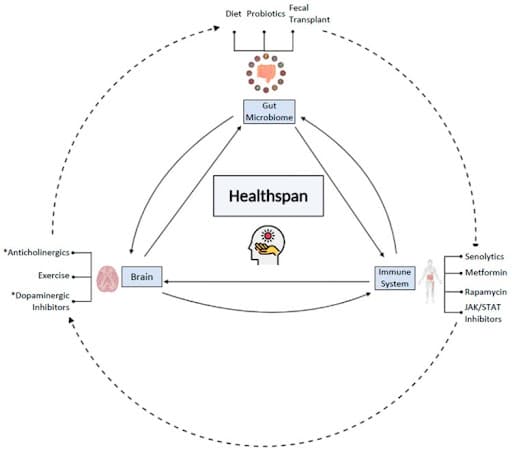
One of the key areas microbiome researchers are exploring is gut plasticity, asking whether researchers can use their newfound knowledge of the gut microbiome to design interventions that not only treat disease, but improve healthspan [1]. So far it looks promising: the gut microbiome can trigger systemic inflammation, which is an aspect of “inflammageing” (chronic inflammation that accumulates with age), and dysbiosis has been implicated in several age-associated neurodegenerative diseases [6]. Better understanding of how the microbiome changes with age, what the microbes’ role is in the bidirectional communication between the gut and brain (the gut-brain axis), and how these systems interact with our immune defenses, could enable targeted interventions that improve our lifespans and healthspans.
Figure 3. The gut microbiome, immune system, and brain all cross-communicate and can be modulated by interventions to improve healthspan and lifespan. Some interventions may more directly impact one system, some may impact indirectly, such as the direct impact of diet and fecal transplant, and the indirect impact of exercise on the gut microbiome. Nonetheless, the gut microbiome, the brain, and the immune system are all connected to one another, and along with age, change in one system can subsequently impact the other systems, ultimately impacting healthspan and lifespan as well. Moreover, the impact of individuals and combinations of interventions on the key players of healthspan and lifespan are still being explored [6].
To find out more details and learn more on how healthy microbiomes can be studied in animal models, please download our white paper at the link below.
In this white paper you will learn:
- The external effects (diet, environment, birth) on microbiome
- Microbiome products and therapies
- The functional foods and medicinal foods markets & the companies making them
- Regulatory concerns
- Traditional and alternative models to research microbiome
- More…
References
- Ursell LK, Metcalf JL, Parfrey LW, Knight R. Defining the Human Microbiome. Nutr Rev. 2012;70: S38.
- Donaldson GP, Lee SM, Mazmanian SK. Gut biogeography of the bacterial microbiota. Nat Rev Microbiol. 2016;14: 20-32.
- Rivière A, Selak M, Lantin D, De Vuyst L. Figure 1: Spatial distribution and concentrations of bacteria along the. 28 Jun 2016 [cited 27 Oct 2021]. Available: https://www.researchgate.net/figure/Spatial-distribution-and-concentrations-of-bacteria-along-the-gastrointestinal-tract-of_fig4_304577663
- Grover K, Gregory S, Gibbs JF, Emenaker NJ. A discussion of the gut microbiome’s development, determinants, and dysbiosis in cancers of the esophagus and stomach. J Gastrointest Oncol. 2021;12: S290-S300.
- Figueiredo ART, Kramer J. Cooperation and Conflict Within the Microbiota and Their Effects On Animal Hosts. Front Ecol Evol. 2020;0. doi:10.3389/fevo.2020.00132
- Narasimhan H, Ren CC, Deshpande S, Sylvia KE. Young at Gut-Turning Back the Clock with the Gut Microbiome. Microorganisms. 2021;9. doi:10.3390/microorganisms9030555





